2422
A Highly Water-soluble Hemin-Based Polymeric Contrast Agents for Magnetic Resonance Imaging1Departments of Radiology, West China Hospital, Sichuan University, Cheng du, China, 2Department of Clinical, Philips Healthcare, China, Wuhan, China, 3Department of Clinical, Philips Healthcare, China, Chengdu, China, 4Department of Clinical, Philips Healthcare, China, Xian, China, 5Departments of Radiology, West China Hospital, Sichuan University, Chengdu, China
Synopsis
Contrast agents play a significant role in clinical MRI and are administered to detect pathology in over a third of clinical scans. Gadolinium (Gd)-based contrast agents (GBCAs) are the mainstream MRI contrast agents used in the clinic. Although GBCAs have shown efficacy and are safe to use with most patients, some GBCAs have a small risk of adverse effects, such as nephrogenic systemic fibrosis (NSF) and gadolinium deposition in the human brain. Therefore, developing novel Gd-free MRI contrast agents are in great demand. Herein, we developed a new type of MRI contrast agent using naturally derived hemin to shorten the T1 relaxation time of water.
Introduction
Magnetic resonance imaging (MRI) has been widely used as a diagnostic method to detect changes in soft tissue due to its exquisite spatial resolution. One of the standard methods to detect pathologies involves the injection of MR contrast agents, such as gadolinium (Gd)-based contrast agents (GBCAs) routinely used for angiography[1]. Although GBCAs are currently the mainstream clinical MRI contrast agents, some GBCAs have shown possible long-term adverse effects—nephrogenic systemic fibrosis (NSF) and Gd depositions in the brain [2]. Thus, developing a new MRI contrast agent without Gd is still in great need.With our continued interest in developing new type contrast agents based on porphyrins [3], we employed natural product hemin as a T1 MRI contrast agent. Hemin is a natural Fe3+-porphyrins of hemoglobin and may be useful as an MRI contrast agent because hemin is a high spin complex [4]. However, it is virtually insoluble under physiological conditions. Here, we synthesized water-soluble MRI contrast agents via covalent conjugation between hemin and acrylic acid.
Materials and Methods
Hemin, acrylic acid, azobisisobutyronitrile (AIBN), and N, N-Dimethylformamide (DMF) were commercially available and purchased from Aladdin. Hemin polymer was prepared according to previous literature [5]. Hydrodynamic diameter and Zeta potential of hemin polymer were characterized by dynamic light scattering (Nano ZS 90, Malvern, UK). MRI experiments are performed on 7T MRI (Bruker Biospec, Ettlingen, Germany).Results and Discussion
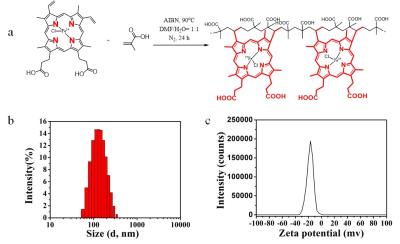
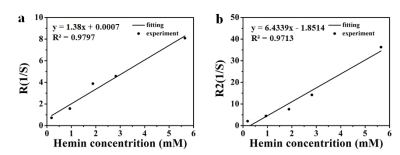
As shown in Figure 1a, this functionalized polymer consisted of hemin and acrylic acid. A facile approach uses natural hemin as the cross-linker in the polymer via a radical-induced polymerization. The presence of a double bond in the 2-methacrylic acid structure as the main monomer and the two vinyl groups in hemin as the copolymer is key for the polymerization. Dynamic light scattering (DLS) measurement shows that the hydrodynamic diameter of polymer is about 122 nm (Figure 1b), and Zeta potential is about -5 mV (Figure 1c).Further measurement of T1 or T2 relaxation time is performed on 7T MRI (Bruker Biospec, Ettlingen, Germany). The test is performed using different concentrations of hemin polymer in PBS. The concentration is confirmed by UV spectral. As shown in figure 2, hemin polymer have an r1 of 1.3 s−1·mM−1 and r2 of 6.4 s−1·mM−1 (Figure 2a and b). To quantitatively evaluate the T1 contrast power of hemin polymer and differentiate it with previously reported agents[4], we compare the r1 and r2 values of hemin polymer and other types of T1 contrast agents. As shown in table 1, hemin polymer has an r2/r1 of 4.9, which is 2.8 times higher than the r2/r1 of the clinical available MRI agent Gd-DTPA, but 2.2 times higher than other commercially available SPION-based ferumoxytol.
Conclusion
In this work, we present a novel Gd-free MRI contrast agent was using clinically compatible agents (hemin). This represents a new class of polymeric MRI contrast agents with good water solubility and T1 relaxation enhancement effect. The polymeric materials could be used as a platform for targeted T1 weighted imaging for a wide variety of medically important receptors, enzymes, and transporters. More work is ongoing for in vivo MR imaging using hemin polymer.Acknowledgements
No acknowledgement found.References
[1] E. Boros, E. M. Gale, P. Caravan, MR imaging probes: design and applications, Dalton Transactions, 2015, 44, 4804-4818;
[2] Zhang X, Yuan Y, Li S, et al. Free-Base Porphyrins as CEST MRI Contrast Agents with Highly Upfield Shifted Labile Protons. Magnetic Resonance in Medicine, 2019, 82, 577-585.
[3] He W, Oliver T, Michael G, et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents, Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(9), 2325-2330.
[4] Simin B, Huguette S, Jessica M, et al. Synthesis and Characterization of Temperature Sensitive and Chemically Cross-Linked Poly(N‑isopropyl acrylamide)/Photosensitizer Hydrogels for Applications in Photodynamic Therapy, Biomacromolecules 2018, 19, 1592−1601.
[5] Heinz P, Nihan G, Weiqiang D, et al. Labeling of Collagen Type I Templates with a Naturally Derived Contrast Agent for Noninvasive MR Imaging in Soft Tissue Engineering, Advanced Healthcare Materials. 2018, 1800605.