2420
Characterization of Self-assembled G4·K+ Hydrogel using CEST MRI
Xiaoling Gong1, Xiaoxiao Zhang2, Xiaoyong Zhang2, and Bing Wu1
1Department of Radiology, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China, Chengdu, China, 2Philips Healthcare, Beijing, China
1Department of Radiology, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China, Chengdu, China, 2Philips Healthcare, Beijing, China
Synopsis
CEST MRI is a novel molecular imaging technique in which the contrast is generated by the dynamic exchange between the exchangeable proton and water. Self-assembled G4·K+ Hydrogel can be fully characterized by its intrinsically endowed CEST contrast.
Introduction
Self-assembly is a powerful way to make new materials, such as supramolecular hydrogels. Hydrogels have been widely applied in drug delivery, cell culture, and tissue engineering. In the context of tissue engineering for biological application, the ability to monitor and track tissue-engineered constructs is highly desirable to assess their integrity, remodeling, and degradation1,2. To render real-time reporting the integrity of tissue engineering in vivo, imaging-guided theranostic systems have been widely explored to incorporate hydrogel with different imaging modalities such as positron emission tomography (PET), single-photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), and ultrasonic and fluorescence imaging. However, safety and stability concerns associated with their toxicities or radioactivities have promoted the development of new generations of a label-free method to assess hydrogel in vivo. CEST imaging represents an attractive alternative strategy because the MR contrast is generated from bioorganic molecules containing exchangeable protons such as −OH, −NH, and −NH2 3. One unbeatable advantage of using CEST MRI for pursuing tissue engineering is that with the intrinsically endowed CEST contrast, extensive chemical labeling of bioactive molecules can be avoided4. In this context, we report on the pioneering use of a natural nucleotide, guanosine (G), as both a CEST MRI contrast agent and molecular building units to create supramolecular filament hydrogels. The use of G to create self-assembling G4 hydrogels represents an effective strategy to create new materials for tissue engineering. The self-assembled hydrogels show a well-defined CEST peak at 6.0 ppm frequency offset from the resonance of water.Materials and Methods
Guanosine, Potassium hydroxide, and boric acid were commercially available and purchased from Aladdin. G4·K+ hydrogel was performed according to previous literature [2]. CEST MRI was performed on 9.4T MRI (Bruker Biospec, Ettlingen, Germany).Results and Discussion
As shown in Figure 1a, this self-assembled hydrogel was easily prepared through one-pot reaction. Borate anion, vicinal diol on guanosine, together with K+, are crucial for gelation. The 0.5 equivalent borate is critical for self-assembly and increased hydrogel lifetime, consistent with two borate diesters (Figure 1) being central to the gel structure. With the G4 hydrogel and guanosine in hand, CEST MRI was performed using different saturation power. While guanosine does not dissolve well in water, the hydrogel is homogeneous without any guanosine precipitation. As shown in figure 2a, At neutral pH, G4 hydrogels produced well defined CEST peak at 6 ppm from water. The peak at 6 ppm is shifted well past labile protons found in well-characterized tumor metabolites which resonated between 1-4 ppm from water, making this signal well suited for specific probe detection. We measured the proton exchange rates using a QUESP experiment (Figure 3) [4]. G4·K+ hydrogel has a quiet slow exchange rate (0.42 ks-1 ) at physiological pH values (pH = 7.0), and the ksw is below the chemical shift difference at 9.4 T (∆ω = 2,400 Hz), placing these in the slow exchange NMR regime and making this agent well suited for CEST imaging.Conclusion
we have developed an MRI-based self-assembled hydrogel system that directly uses natural nucleotide as both the molecular building units and MRI contrast agents. With the inherent CEST MRI signal afforded by assembled guanosine at 6 ppm, the G4·K+ hydrogel can be well characterized without any label which may affect the stability. More work focused on tissue engineering is ongoing for the in vivo MR imaging using G4 hydrogel.Acknowledgements
No acknowledgement found.References
[1] Hirst AR, Escuder, B.; Miravet, J. F.; Smith, D. K. High-Tech Applications of Self-Assembling Supramolecular Nanostructured Gel-Phase Materials: From Regenerative Medicine to Electronic Devices, Angew. Chem., Int. Ed. 2008, 47, 8002; [2] Gretchen MP, Luke PS, Taylor NP, et al, A G4·K+ Hydrogel Stabilized by an Anion, J. Am. Chem. Soc. 2014, 136, 12596−12599; [3] McMahon MT, Gilad AA, Bulte JWM, van Zijl PCM. Chemical Exchange Saturation Transfer Imaging: Advances and Applications. Singapore: Pan Stanford Publishing; 2017. 479 p; [4] Lye LL, Yuguo L, Xinpei M, et al One-Component Supramolecular Filament Hydrogels as Theranostic Label-Free Magnetic Resonance Imaging Agents, ACS Nano 2017, 11, 1, 797–805 [5] McMahon MT, Gilad AA, Zhou J, Sun PZ, Bulte JW, van Zijl P. Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): pH calibration for poly-L-lysine and a starburst dendrimer. Magnetic resonance in medicine, 2006, 55(4): 836-847.Figures
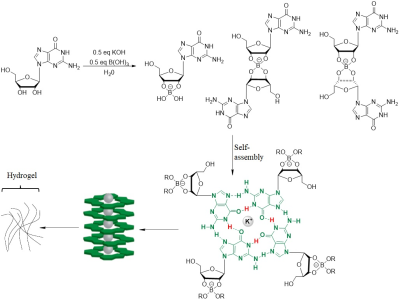
Figure
1. In the presence of
KB(OH)4, guanosine (1) forms borate monoester 4 and diastereomeric
borate diesters, then Self-assembly of borate diesters into a structure
containing stacked G4 quartets.
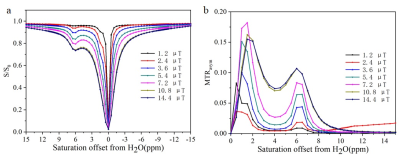
Figure 2. Characterizing the CEST
properties of G4 quartets. (a) Z-spectrum; (b) MTRasym
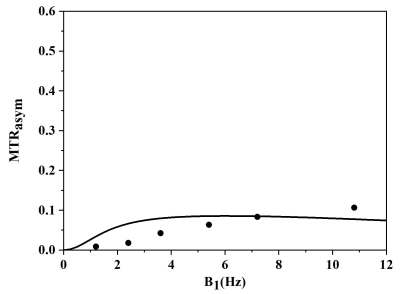
Figure 3. QUESP data of G4
quartets
DOI: https://doi.org/10.58530/2022/2420