2419
Selective galactose culture condition reveals distinct metabolic signatures in complex I and V deficient human skin fibroblasts by 1H HR-MAS NMR1Magnetic Resonance Methodology, Institute of Diagnostic and Interventional Neuroradiology, University Bern, Bern, Switzerland, 2Institute of Clinical Chemistry, University Hospital Bern, Bern, Switzerland, 3Graduate School for Cellular and Biomedical Sciences, University of Bern, Bern, Switzerland, 4Department of Pediatric Endocrinology, Diabetology and Metabolism, University Children’s Hospital of Bern, Bern, Switzerland
Synopsis
Mitochondrial respiratory chain defects present as highly heterogeneous disorders which cannot be unambiguously diagnosed using standard laboratory methods. In this study we observed the biochemical consequences of complex I and complex V deficient human skin fibroblasts when cultivated under galactose stress condition compared to glucose based cell culture condition. We investigated extracellular flux using Seahorse XFe96 cell analyzer and assessed the metabolome fingerprints using High Resolution Magic Angle Spinning NMR. The selective culture method reveals CI and CV defect-specific changes in metabolites associated with the TCA cycle, malate aspartate shuttle and choline metabolism.
Introduction
Galactose based culture decreases activity of anaerobic glycolysis and induces aerobic energy production1. It has been shown that galactose based media lead to an increase in mitochondrial oxidative metabolism and better discriminating metabolic signatures in mitochondrial dysfunctions2. We have previously shown that a TCA cycle defect (pyruvate dehydrogenase defect) showed differently altered metabolites compared to a respiratory chain defect (complex I defects)3. We were curious whether the metabolic profiles of even structurally nearby defects such as CI and CV, both within the respiratory chain, were distinguishable from each other and from controls using NMR based metabolite profiles. Within the respiratory chain, CI deficiency impairs the oxidation of NADH to NAD+ and CV deficiency limits the ATP generation4. We hypothesise that these different biochemical functions of CI and CV generate different metabolic signaling in dysfunction. Studying the metabolic impact of different respiratory chain defects in combination with routine extracellular flux analysis can help identify and characterize signaling cascades caused by defects and therefore help to reveal yet unknown relationships between mitochondrial dysfunction and secondary metabolic alterations.Methods
Cell culturing: We studied fibroblasts derived from three controls, two patients with complex I (CI; NDUFS3, NDUFS4) and two patients with complex V (CV; MT-ATP6, MT-ATP6/8) deficiency. Cells were grown for 18 hours under standard culture condition (modified DMEM with 5.5mM glucose, 10% dialyzed FCS) or were exposed to galactose based stress medium (replacing glucose with 10mM galactose).NMR Spectroscopy: Sample preparation for 1H-HR-MAS NMR was performed as described previously3. Each sample was lysed after collection and heated (70°C, 20min). On average 2.2 million cells in D2O-based PBS were inserted into 4mm rotors using a 50µl insert. HR-MAS NMR experiments were performed on a 500 MHz Bruker Avance II spectrometer. A PROJECTpr pulse sequence5 was used for acquiring 1H-NMR spectra. Temperature 275K, spinning speed 5kHz, TE 120ms, 768 transients. Spectral processing included line-broadening, FFT, phasing and baseline-correction. Chemometrics and individual peak analysis was performed with MatLab, PLS_Toolbox (Eigenvector Research, Inc.) and Excel, using PQN-normalized individually sized buckets.
Seahorse XFe96 metabolic flux analysis: We investigated metabolic flux in unbuffered culture medium using a Seahorse XFe96 cell analyzer. The oxygen consumption rate (OCR), an assessment of aerobic mitochondrial function, and extracellular acidification rate (ECAR), a detection of extracellular acid production, were measured simultaneously.
Study design: The presented data are part of an ongoing comprehensive study on numerous different metabolic defects (in triplicates and under standard and stress culture condition) with the long-term aim to establish 1H HR-MAS NMR as screening method and to gain insight into the pathophysiology of these diseases. Here we present the preliminary results on three controls, two CI defective and two CV defective cell lines. In detail: For Ctrl1, Ctrl2, Ctrl3, CI (NDUFS3), CI (NDUFS4), CV (MT-ATP6) and CV (MT-ATP6/8) 3, 4, 2, 1, 3, 3 and 2 replicates were included, respectively under glucose condition and 2, 0, 3, 1, 3, 3 and 3 replicates under galactose condition.
Results and Discussion
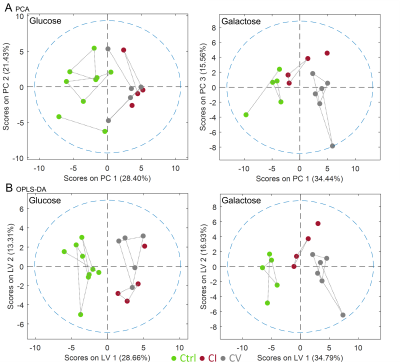
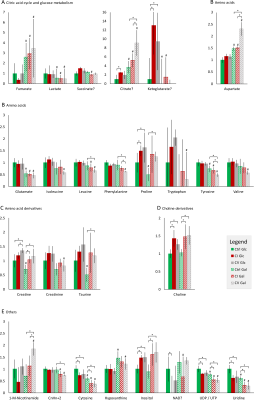
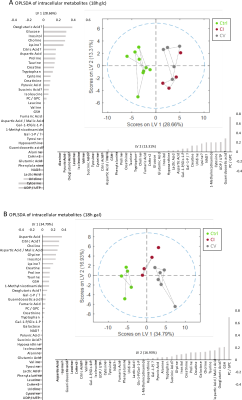
Bioenergetic analysis: Incubation of all measured cell lines in galactose condition led to an expected shift from glycolytic towards aerobic metabolism (Figure 1A). The mean basal respiration of the pooled control cell lines was significantly higher than the mean respiration of both CI and CV defective cell lines in glucose and in galactose condition. The respiratory spare capacity of CI deficient cells was significantly lowered compared to controls in glucose condition but not in galactose stress condition and was significantly lowered compared to CV deficient cells in both glucose and galactose condition. (Figure 1B).NMR spectroscopy: Unbiased PCA of overall 39 assigned intracellular metabolites revealed separation of controls and defective cell lines along PC1 for both conditions (Figure 2A). However, CI and CV defects overlapped in glucose condition, but were mostly separated in galactose stress condition. Orthogonal partial least squares discriminant analysis (OPLS-DA) of the 39 metabolites showed similar to PCA separation along LV1 under glucose and galactose based condition (Figure 2B). While in glucose the metabolite pattern of CI defects overlapped with CV defects, this was not the case under galactose condition (see Figure 4 for OPLSDA loadings). A total of 27 out of 39 intracellular metabolites were found to be changed significantly between controls, CI and CV defects for both conditions with 18 and 26 metabolites being different under glucose and galactose condition, respectively (Figure 3). This suggests that CI and CV deficient cells can be distinguished from each other solely based on distinct metabolic signatures.
While choline and uridine were increased in both respiratory chain defects, currently available data reveal metabolites associated with the TCA cycle, malate aspartate shuttle and phenylalanine catabolism (citrate, aspartate, phenylalanine, and tyrosine) as potential biomarkers separating mitochondrial CV from CI defects in galactose condition.
Conclusion
This preliminary metabolomics HR-MAS NMR study indicates the benefit of using selective versus standard culture conditions providing better separation and insights into disturbed oxidative metabolism by respiratory chain disorders. First results confirm CI and CV defect specific metabolic adaptations in human fibroblasts. Other mitochondrial defects will be included to characterize whether the identified metabolites may be relevant as biological markers in the studied defects or other disorders.Acknowledgements
Supported by the Swiss National Science Foundation (SNSF #310030_192691).References
1. Bustamante, E. & Pedersen, P. L. High aerobic glycolysis of rat hepatoma cells in culture: role of mitochondrial hexokinase. Proc. Natl. Acad. Sci. U. S. A. 74, 3735–3739 (1977).
2. Aguer, C. et al. Galactose Enhances Oxidative Metabolism and Reveals Mitochondrial Dysfunction in Human Primary Muscle Cells. PLoS One 6, e28536 (2011).
3. Hertig, D. et al. Selective galactose culture condition reveals distinct metabolic signatures in pyruvate dehydrogenase and complex I deficient human skin fibroblasts. Metabolomics 15, 32 (2019).
4. Sousa, J. S., D’Imprima, E. & Vonck, J. Mitochondrial Respiratory Chain Complexes. Subcell. Biochem. 87, 167–227 (2018).
5. Aguilar, J. A., Nilsson, M., Bodenhausen, G. & Morris, G. A. Spin echo NMR spectra without J modulation. Chem. Commun. 48, 811–813 (2012).
Figures