2418
Diffusion MRI protocol optimization for ex vivo mouse whole-brain tractography at 100μm isotropic resolution1Centre de Résonance Magnétique des Systèmes Biologiques, UMR5536, CNRS, Bordeaux, France, 2Groupe dImagerie Neurofonctionnelle, Institut Des Maladies Neurodegeneratives, UMR5293, CNRS, CEA University of Bordeaux, CNRS, Bordeaux, France
Synopsis
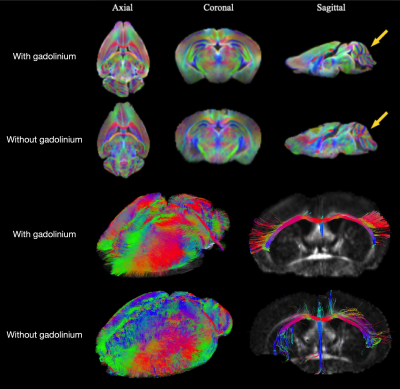
A protocol, including Gadolinium-DOTA perfusion or immersion and Diffusion-Weighted multi-shot Spin-Echo EPI was developed to obtain 100μm isotropic resolution tractogram on ex vivo mouse brain. A 3mM Gd-DOTA concentration combined with 16 EPI shots at short TR (400 ms) allows to whole-brain tractograms of excellent quality and reproducible on the 15 mouse brains analyzed.
Introduction
MRI is widely used to understand brain architecture and function in humans. Advances in instrumentation and sequences have made fMRI and dMRI methods usable for studying rodent models. Studying these models is of great importance since they mimic many neuropathologies, are reproducible, and can be combined with more invasive studies. However, routine rodent brain connectomics is still challenging. It is necessary to generate images with high spatial resolutions (at least of the order of 100µm) while maintaining a high signal-to-noise ratio [1,2]. In the case of diffusion MRI and tractography, the images are essentially acquired ex vivo. In this case, it is also necessary to acquire images with a high diffusion value (> 2000mm2/s), sample an adequate number of gradient angles, and maintain a reasonable scan time of less than one night per brain. The use of high magnetic fields (≥7T) also leads to numerous artifacts that must be limited. Our work aims to propose a standardized protocol for acquiring diffusion images on ex vivo mouse brain at 100 μm3 spatial resolution and 60 gradient angles. For this, we optimized a SE-EPI diffusion sequence and the protocol for using a T1 contrast agent.Methods
Brain Preparation.Two methods of Gd-DOTA incorporation in the mice brain were used: perfusion-fixation and simple immersion-fixation with Gd-DOTA at various concentrations (0, 0.625, 3, and 6.25 mM).
For imaging, each brain (N=15) was placed in a plastic tube with a 3D printed support, including a bubble trap. The brain was then installed at the center of the NMR coil, in the rostrocaudal direction, at 25°C.
MR System.
All imaging experiments were performed using at 7T Bruker Biospec 70/20 system (Ettlingen, Germany) and a 12 cm gradient insert capable of 660 mT/m. A volume resonator was used for excitation and a 4-element mouse brain phased-array surface coil for signal reception. Sequences T1 and T2 were measured on the extracted brain with an Inversion Recovery-RARE and MSME (Multi-Slice Multi-Echo) sequences.
Ex vivo Diffusion Imaging.
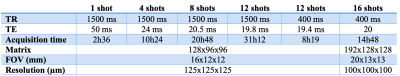
The DWI (Diffusion-Weighted Imaging) sequence was a 3D Spin-Echo EPI using 60 different directions at b = 3000mm2/s and five b0 images. Various parameters were modified to optimize the protocols: repetition time (TR), numbers of EPI shot, and echo time (TE) (cf. Table 1).
Image processing for DTI Imaging.
DWI data pre-processing and analysis used a combination of tools from the FMRIB software library, Advanced Normalization Tools (ANTs), and the SCILPY library [3]. Pre-processing steps were to build a fractional anisotropic (FA) map [4] (ref) from the raw DWI data to create a brain mask and homogenize the diffusion signal. Each FA map was aligned to the Australian Mouse Brain Mapping Consortium (AMBMC) atlas [5] using ANTs. Brain-wide diffusion metrics were therefore calculated on the 15 AMBMC-normalized brains.
Results
Table 2 shows the values of T1 and T2 of the brain as a function of the concentration of Gadolinium perfused. Similar results were obtained with the Gd-DOTA immersion-fixation protocols, but at least after 2 weeks of immersion. After 30 days of immersion in a PBS solution, the brain shows a good clearance of Gadolinium. The T1 brain is similar to that of a brain not incubated with Gd-DOTA.The DW SE-EPI sequence was optimized to be less sensitive to the susceptibility effects by increasing the number of shots. Unsurprisingly the susceptibility artifacts decreased with this number but increased the total acquisition time.
Finally, the best compromise we identified is :
- Infusion (or immersion at least two weeks) with 3 mM Gadolinium
- A DW SE-EPI sequence of 12 or 16 shots with a TE/TR = 20/400 ms.
- 60 directions of gradient angles for a total acquisition time of 14H48 for 100μm3 resolution.
Discussion
The use of Gadolinium can be performed in two ways, perfusion or immersion. While the immersion method is longer and requires at least 15 days of incubation, it can be performed after the animal's sacrifice. It is important to note that in both cases, the process is quasi-reversible. Gadolinium can be removed from the brain to not interact with other products or methods (e.g., CLARITY or DISCO techniques). A multi-shot EPI sequence is less sensitive to artifact than a single-shot sequence but increases acquisition time. To counter this problem, adding Gadolinium with an optimal concentration (3mM) reduces the sequence TR without losing signal-to-noise ratio and a too-high T2 decrease.Conclusion
In conclusion, we have optimized a protocol for 100μm3 diffusion imaging with 60 directions in an acquisition time of less than 15H. Thus, an analysis can be launched almost every night on the MRI scanner. The generated tractograms are of excellent quality and be reproducible on the 15 mouse brains analyzed.Acknowledgements
This work was supported by Nouvelle Region Aquitaine within the "Imagerie ultra-rapide du connectome: application pré-clinique" project and CNRS.References
[1] Optimizing Diffusion Imaging Protocols for Structural Connectomics in Mouse Models of Neurological Conditions. Anderson RJ, Long CM, Calabrese ED, Robertson SH, Johnson GA, Cofer GP, O'Brien RJ, Badea A. Front Phys. 2020 Apr;8:88. doi: 10.3389/fphy.2020.00088.
[2] A time-course study of actively stained mouse brains: Diffusion tensor imaging parameters and connectomic stability over 1 year. Xiao J, Hornburg KJ, Cofer G, Cook JJ, Pratson F, Qi Y, Johnson GA. NMR Biomed. 2021 Sep 23:e4611. doi: 10.1002/nbm.4611.
[3] https://github.com/scilus/scilpy/
[4] Dhollander T, Smith RE, Tournier J-D, Jeurissen B, Connelly A (2015) Time to move on: an FOD-Based DEC map to replace DTI's trademark DEC FA. In: 23rd ISMRM Annual Meeting. Toronto, Canada.
[5] Ullmann JFP, Watson C, Janke AL, Kurniawan ND, Reutens DC (2013) A segmentation protocol and MRI atlas of the C57BL/6J mouse neocortex. NeuroImage 78:196-203.
Figures