2417
Probing compartment-specific diffusion in the brain with 13C diffusion-weighted MRS of hyperpolarized pyruvate and lactate: initial results1MIRCen / Neurodegenerative Diseases Laboratory, CEA, Fontenay-aux-Roses, France, 2Department of Physical Therapy and Rehabilitation Science, UCSF, San Francisco, CA, United States, 3Department of Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States
Synopsis
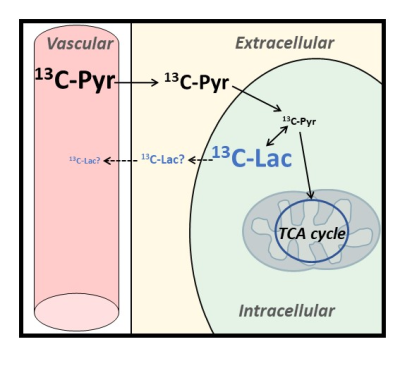
Here we propose to use 13C diffusion-weighted MRS of hyperpolarized pyruvate and lactate to probe diffusion properties in distinct compartments of the mouse brain, tentatively extracellular (ECS) and intracellular spaces (ICS). Hyperpolarized 13C-pyruvate is injected intravenously and taken up by the brain before entering cells, where it is quickly converted to lactate, so that 13C-pyruvate concentration in ICS must remain low, while newly produced 13C-lactate should remain predominantly intracellular during the relatively short measurement time. We measure faster diffusion for pyruvate even after removing IVIM effect, suggesting faster diffusion in the ECS, and slow release of lactate in the ECS.
INTRODUCTION
Elucidating how diffusion properties differ between brain intracellular (ICS) and extracellular spaces (ECS) has been driving abundant research. However, no consensus has been reached so far. Indeed, water molecules as detected by diffusion-weighted (DW) MRI are ubiquitous and thus do not specifically inform on any compartment. Unlike water, most brain metabolites as detected by DW-MRS are intracellular, and can hence provide specific information on ICS diffusion. Unfortunately, no ECS-specific endogenous marker exists, making the exploration of ECS diffusion properties more challenging. Various approaches have been proposed, such as theoretical considerations1, numerical simulations2 or intracranial injection of exogenous probes3-5. Here we propose another approach, based on 13C DW-MRS of hyperpolarized pyruvate and lactate. Hyperpolarized 13C-pyruvate is injected intravenously and taken up in the brain before entering the cells, where it is very quickly converted to lactate, so that 13C-pyruvate concentration in ICS must remain low. In contrast, 13C-lactate (i.e. just created from hyperpolarized 13C-pyruvate) should remain predominantly intracellular, provided 13C-lactate release in ECS via monocarboxylate transporters is slow compared to measurement time (Figure 1).METHODS
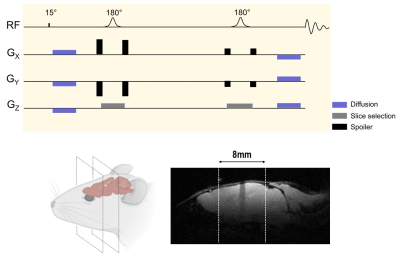
Acquisitions were performed on a 3 Tesla horizontal system (Bruker) equipped with a dual-tuned 1H/13C surface coil (Neos Biotech) attached to a custom-made 3D printed holder. Two experiments were performed. 24 μL of [1-13C]pyruvate was co-polarized for ~1h in a Hypersense polarizer (Oxford Instruments), and rapidly dissolved in 4.5-mL heated buffer (80 mM NaOH in PBS). 300 μL of the hyperpolarized solution was then injected over 14 seconds via a tail vein catheter. MRS acquisitions started 12 seconds after the start of pyruvate injection. MRS was performed using a single-slab LASER selection, surrounded by pair of diffusion-sensitizing gradients of opposite polarities (Figure 2). The b-values (b=0.02, 0.5 and 3 ms/µm²) were looped across repetitions (TR=2 second) until the end of the acquisition (total duration=80 seconds). Signal attenuation between 0.02 and 0.5 ms/µm² is expected to mostly reflect IVIM, while attenuation from 0.5 to 3 ms/µm² should allow calculating an “IVIM-free” apparent diffusion coefficient (ADC).RESULTS
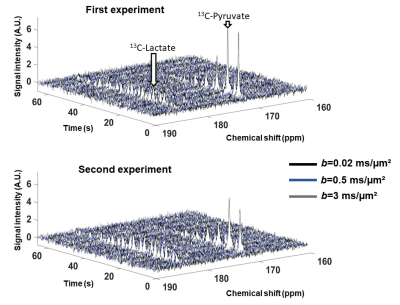
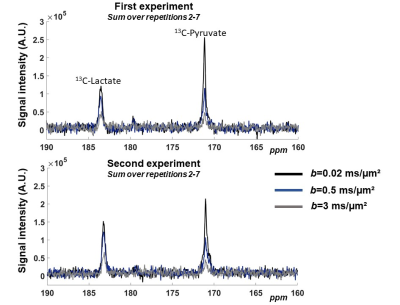
Spectral time-courses acquired during the two experiments are shown in Figure 3. For each b-value, spectra acquired between the 2nd and the 7th repetitions (i.e. when both pyruvate and lactate peaks are above noise) were summed, resulting in one averaged spectrum for each b-value (Figure 4).One striking feature is the very strong decrease of 13C-pyruvate signal between 0.02 and 0.5 ms/µm², in line with a very large vascular fraction of injected pyruvate. In contrast, 13C-lactate attenuation is very moderate, suggesting that newly created lactate has not released in the blood yet.
The other striking feature is the different attenuation for pyruvate and lactate between 0.5 and 3 ms/µm², which is again stronger for pyruvate, corresponding to ADCPyr=0.45 µm²/ms and ADCLac=0.28 µm²/ms for the first experiment, and ADCPyr=0.53 µm²/ms and ADCLac=0.38 µm²/ms for the second experiment. Hence, 13C-pyruvate and 13C-lactate indeed appear to reflect diffusion in different compartments imposing different diffusion properties (since molecular weights and free diffusion coefficients of pyruvate and lactate are otherwise mostly identical).
DISCUSSION
Lactate is synthetized in ICS from pyruvate, hence hyperpolarized lactate diffusion must predominantly reflect diffusion in ICS, provided it does not too quickly redistribute between ICS and ECS after its release in ECS via monocarboxylate transporters. Here we cannot tell to what extent 13C-lactate has been released in ECS, but experiments may be designed to minimize the sensitivity to ECS (e.g. by performing 90° excitation on the lactate peak, thus killing all longitudinal magnetization, so that observed signal can only come from lactate produced during the last TR, leaving little time for release in ECS). However, there is little doubt that 13C-pyruvate is mostly in ECS and 13C-lactate mostly in ICS.ADC reported here are unexpectedly high. Indeed, based on ADC measurements of sucrose injected in ECS (where ADC was found to be ~Dfree/3, with Dfree measured in phantom at 37°C5, one would rather expect ADC~0.35 µm²/ms for pyruvate in ECS. Similarly, based on ADC measurements of endogenous intracellular metabolites (where ADC is usually found to be ~Dfree/6, with Dfree measured in phantom at 37°C5), one would expect ADC~0.18 µm²/ms for lactate in ICS. We think that our measurements might be biased towards larger ADC values due to bulk motion. However, such bulk motion is not expected to affect the absolute ADC difference. Incidentally, the absolute difference between measured ADC for pyruvate and lactate is 0.15-0.17 µm²/ms, which is almost exactly the expected difference between ADC in ECS and ICS for these molecules, as calculated above. This reinforces the idea that hyperpolarized pyruvate and lactate are indeed highly specific markers of ECS and ICS.
CONCLUSION
In this proof-of-concept study, DW-signal attenuations of hyperpolarized 13C-pyruvate and 13C-lactate exhibit different properties in the mouse brain, with strong IVIM effect for pyruvate but not for lactate, and “IVIM-free” ADC for pyruvate faster than for lactate suggesting faster ADC in ECS than in ICS. Beyond studying diffusion, our approach may allow assessing the dynamics of lactate release in ECS (e.g. by performing low-flip angle versus 90° excitation of lactate, and measuring how lactate diffusion varies between both conditions), which might prove invaluable to characterize lactate metabolism and shuttling.Acknowledgements
This work was funded by the ISMRM Research Exchange Grant Program (to MV), the Fulbright – Paris Saclay University Doctoral Grant Program (to MV), NIH R01NS102156 (to MMC), NMSS research grant RG-1701-26630 (to MMC), the Dana Foundation (to MMC), and the European Research Council (grant number 818266 awarded to JV).References
1. Novikov DS, Jensen JH, Helpern JA, Fieremans E. Revealing mesoscopic structural universality with diffusion. Proc Natl Acad Sci U S A 2014;111(14):5088-5093.
2. Ginsburger K, Matuschke F, Poupon F, Mangin JF, Axer M, Poupon C. MEDUSA: A GPU-based tool to create realistic phantoms of the brain microstructure using tiny spheres. Neuroimage 2019;193:10-24.
3. Duong TQ, Sehy JV, Yablonskiy DA, Snider BJ, Ackerman JJ, Neil JJ. Extracellular apparent diffusion in rat brain. Magn Reson Med 2001;45(5):801-810.
4. Kunz N, da Silva AR, Jelescu IO. Intra- and extra-axonal axial diffusivities in the white matter: Which one is faster? Neuroimage 2018;181:314-322.
5. Vincent M, Gaudin M, Lucas-Torres C, Wong A, Escartin C, Valette J. Characterizing extracellular diffusion properties using diffusion-weighted MRS of sucrose injected in mouse brain. NMR Biomed 2021;34(4):e4478.
Figures