2416
Qualitative assessment of Ex-vivo Manganese-Enhanced MRI of gyrencephalic brains
Nathalie Just1, Martine Batailler2, Jean-Philippe Dubois2, and Martine Migaud2
1DRCMR, Copenhagen University Hospital - Amager and Hvidovre, Hvidovre, Denmark, 2INRAE, Nouzilly, France
1DRCMR, Copenhagen University Hospital - Amager and Hvidovre, Hvidovre, Denmark, 2INRAE, Nouzilly, France
Synopsis
In-vivo Manganese-Enhanced MRI (MEMRI) studies have shown important potential in rodents for the delineation of cytoarchitectural brain features and of functional details. Ex-vivo MEMRI on the other hand offers the possibility to investigate brain microstructure and function with improved spatial resolution and no motion artefacts. Here, ex-vivo lamb brains were immersed in a highly concentrated MnCl2 solution for a month and revealed interesting cytoarchitectural features already 24 hours after immersion and up to 3 months after the end of immersion on MPRAGE images at 3T. A novel ex-vivo MEMRI approach is proposed, which could benefit MEMRI translation to human studies.
Introduction
In the rodent’s lissencephalic brain, manganese-enhanced MRI (MEMRI) demonstrated to be an unavoidable technique for the delineation of cytoarchitectural features at unprecedented spatial resolutions in-vivo (1).One of the main drawbacks of manganese ions (Mn2+), is their toxicity especially at high doses. To observe anatomical and functional information without artefacts and at high spatial resolutions, ex-vivo MEMRI after in-vivo Mn2+ administration have been privileged but only a few studies have been conducted (2).In particular, difficulties to maintain the Mn2+ contrast in fixed tissues have been reported (3). Here, an ex-vivo MEMRI quqlitative exploration of the lambs’ brain microstructure at 3T was performed. Perfused lamb brains were soaked in highly concentrated MnCl2 (> 200 mM) baths and Mn2+ ions were absorbed by brain tissues. The lamb brains were then imaged using T1-weighted techniques.Materials and Methods
All experiments were performed in 4 Ile-de-France (IF) female lambs (4 months old, 30-35kg). Each animal was fasted 24-hours prior to intubation. After immobilization, the lamb was intubated after intravenous administration of a mixture of ketamine and xylazine (20 mg/kg). Each lamb was transported to the MRI room, installed prone on the MRI bed and anaesthesia was immediately switched to 1% ISO in medical air through a respirator (Aestiva, GE Healthcare, Datex-Ohmeda, USA). The respirator allowed continuous control of respiration rates. An oximeter was attached to one of the hind-paws allowing for the control of the partial pressure of oxygen and heart rate. The temperature was controlled through MRI-compatible rectal probe. The duration of each MRI session was limited to 150 minutes for each animal. A continuous physiological follow up of the animal status by a veterinary was performed during experiments. All the lambs were euthanized with an overdose of sodium pentobarbitone (25 mg/kg; Merial, Lyon, France) preceded by an injection of heparin. After euthanasia, animals were decapitated and the heads were perfused through both carotid arteries with 2 L sodium nitrite (1%) in NaCl (0.9%) followed by 4 L of cold paraformaldehyde (4%) in 0.1 M phosphate buffer, pH 7.4. The whole brains were collected. After a 48-hour period of postfixation, each brain was soaked in 20% sucrose for cryoprotection. A bulk solution of manganese chloride (MnCl2) in saline (0.9 %) (200mM, Sigma-Aldrich, St. Louis, MO, USA) was prepared. Three brains were immersed in MnCl2 for one month and returned to a sucrose bath and conserved at 4° C. MRI was conducted 24 hours and after 3 months after MnCl2 immersion. During acquisitions, lamb brains were immersed in a water bath at room temperature. In-vivo and ex-vivo MR imaging were conducted on a 3T whole body MR Scanner (Siemens Verio, Erlangen, Germany). A large flex coil surrounding the entire head for reception and the body coil for transmission were used for in-vivo measurements. For ex-vivo measurements, the 12-element head volume coil was used for transmission. MPRAGE images were acquired in-vivo and ex-vivo with parameters (TR/TE/TI=2500/3.18/900 ms ; Flip angle = 12°; NEX= 2 ; FOV=192 x 192 mm2, Matrix size = 384 x 384 x 256; In-vivo Voxel size = 0.5 x 0.5 x 0.5 mm3 ; Ex-vivo Voxel size = 0.5 x 0.5 x 0.2 mm3). ImageJ was used to visualize and investigate images qualitatively.Results
Without MnCl2, no contrast was seen between different structures of the ex-vivo lamb brain (Fig 1A) whereas ex-vivo brains soaked in MnCl2 for 24 hours (Fig.1B) and in-vivo brains (Fig.1C) revealed important differences. In cortical areas, white matter (WM) to gray matter (GM) contrast was seen in-vivo (Fig 2A) including the stria of Gennari (white arrows). After 24-hours in MnCl2, CSF was replaced by MnCl2-doped liquid (Fig.2B, arrows). After 3 months in sucrose, Mn2+ had diffused in the lamb brains. In cortical areas (Fig 2C), WM appeared as dark and other cell layers displayed gray to white contrast (blue arrows). Within 24 hours, lamb brains soaked in MnCl2 displayed hippocampal layers (Fig.3A and inset) and granulated features in the globus pallidus (Fig.3B and inset) similar to these seen in fiber stained specimens of the sheep brain atlas (Fig.3C) (4). In sagittal and coronal brain slices, 3 months post-immersion, Mn2+ uptake was seen in the hippocampi (white boxes) where different microstructures could correspond to CA3 cells and the dentate gyrus (Fig 4 and Fig.5) by reference to the sheep brain atlas (4). Mn2+ also stained cerebellar structures (yellow boxes, Fig 4 and 5) as well as other structures such as the edges of the thalamus (Th), the hypothalamus and the geniculate nucleus (yellow arrows Fig 4 and 5).Discussion
Despite important susceptibility artefacts, 3D T1-weighted images of ex-vivo lamb brains soaked in a bulk solution of highly concentrated MnCl2 displayed microstructural features of interest such as laminar structures in the hippocampus and cortical layers as soon as 24 hours post-staining and up to 3 months after staining. Quantitative assessments, which will validate these early findings will be performed.Conclusion
A novel methodology for ex-vivo MEMRI observation several months after initial staining was proposed. Although only qualitative for the time being, we expect that this work will open new perspectives for MEMRI in gyrencephalic brains.Acknowledgements
The author acknowledges the help of Martine Batailler and JeaPhilippe Dubois of INRAE Nouzilly, France.References
1. Silva AC, Lee JH, Aoki I, Koretsky AP Manganese-enhanced magnetic resonance imaging (MEMRI): methodological and practical considerations..NMR Biomed. 2004 Dec;17(8):532-43. 2. Sato C, Sawada K, Wright D, Higashi T, Aoki I. Isotropic 25-Micron 3D Neuroimaging Using ex vivo Microstructural Manganese-Enhanced MRI (MEMRI). Front Neural Circuits. 2018 Dec 6;12:110.doi: 10.3389/fncir.2018.00110. eCollection 2018. 3. Huang S, Liu C, Dai G, Kim YR, Rosen BR Manipulation of tissue contrast using contrast agents for enhanced MR microscopy in ex vivo mouse brain. .Neuroimage. 2009 Jul 1;46(3):589-99. doi: 10.1016/j.neuroimage.2009.02.027. 4. The Sheep Brain AtlasJohn I. Johnson, Keith D. Sudheimer, Kristina K. Davis, Garrett M. Kerndt, and Brian M. Winn Radiology Department, Neuroscience Program,and Communications Technology Laboratory,Michigan State University, East Lansing, MFigures
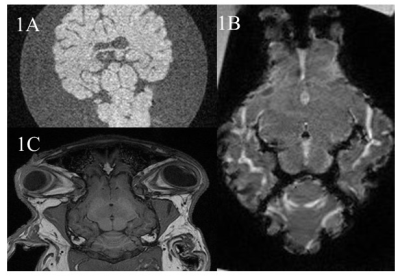
Figure1: Examples of lamb brain MPRAGE images at 3T: A. Ex-vivo immersed in water only. B. EX-vivo 24 hours after immersion in MnCl2 and C. In-vivo
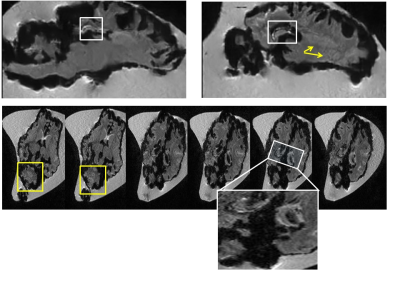
Figure
4: 3 months after immersion in MnCl2,
sagittal and axial MPRAGE images of ex-vivo lamb brains revealed laminar
structures in the hippocampus (White boxes). The hippocampi within an axial
slice where enlarged to show the different contrasts revealed by Mn2+ uptake in
these areas. Yellow boxes and arrows show other features stained by MnCl2
particularly in cerebellar areas.
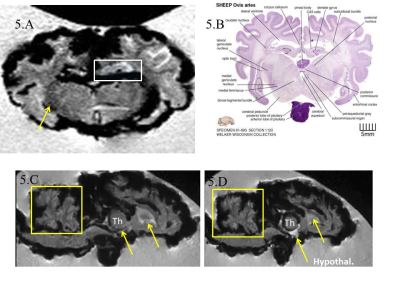
Figure 5: A. the
laminar structures revealed in the hippocampus (white box, axial slice) could
correspond to CA3 cells and the dentate gyrus by reference to the sheep brain
atlas (B). Darker areas (yellow
arrow) show correspondence with the geniculate nucleus differing in contrast to
other areas of the thalamus. C.
Cerebellar structures were also revealed (yellow boxes) as well as other
structures in the thalamus and frontal parts (yellow arrows). D.Mn2+uptake can also be
seen in hypothalamic structures.
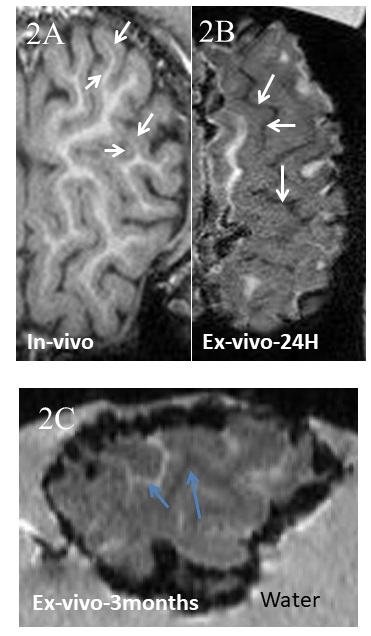
Figure
2: Lamb cortex
MPRAGE A. In-vivo. White arrows indicate the stria of Gennari. B. 24-hour
post-immersion in MnCl2. White arrows
point at areas filled with highly concentrated MnCl2. C. 3 months post-immersion
in MnCl2. Ex-vivo cortical sample of the front brain showing White Matter as
dark and other cortical layers as white (blue arrows).
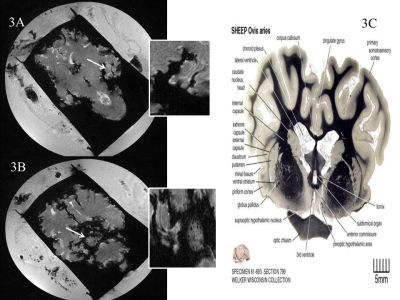
Figure
3: Examples of Lamb brain images immersed
for 24 hours in MnCl2. A. As early as 24-hours post-staining,
laminar structures of the hippocampus (white arrow and enlarged inset). B.
Other structures of interest were revealed such as black dots over the globus
pallidus (white arrow and enlarge inset), which were also seen in C. specimens
of the sheep brain atlas.
DOI: https://doi.org/10.58530/2022/2416