2414
The Digital Brain Bank, an open access platform for post-mortem datasets1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Division of Clinical Neurology, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 3Psychology Department, Emory University, Atlanta, GA, United States, 4Centre for Zoo and Wild Animal Health, Copenhagen Zoo, Copenhagen, Denmark, 5Department of Radiology, University of Chicago, Chicago, IL, United States, 6Department of Complex Trait Genetics, Centre for Neurogenomics and Cognitive Research, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam, Netherlands, 7Department of Child Psychiatry, Amsterdam Neuroscience, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands, 8Medical Research Council Oxford Institute for Radiation Oncology, University of Oxford, Oxford, United Kingdom, 9School of Anatomical Sciences, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa, 10Wellcome Centre for Integrative Neuroimaging, Department of Experimental Psychology, University of Oxford, Oxford, United Kingdom, 11Stem Cell and Brain Research Institute, Université Lyon 1, INSERM, Lyon, France, 12Donders Institute for Brain, Cognition and Behaviour, Radboud University, Nijmegen, Netherlands
Synopsis
We introduce the Digital Brain Bank (open.win.ox.ac.uk/DigitalBrainBank), a digital platform providing open access to curated, multimodal post-mortem neuroimaging datasets. Datasets span three themes; Digital Anatomist: datasets for neuroanatomical investigations; Digital Brain Zoo: datasets for comparative neuroanatomy; Digital Pathologist: datasets for neuropathology investigations. The first release includes 21 distinctive whole-brain diffusion MRI datasets, alongside microscopy and complementary MRI modalities. This includes one of the highest-resolution whole-brain human diffusion MRI datasets ever acquired, whole-brain diffusion MRI in 14 non-human primate species, and one of the largest post-mortem whole-brain cohort imaging studies in neurodegeneration. Our resource facilitates incorporating post-mortem data into neuroimaging studies.
Introduction
Post-mortem MRI provides unique opportunities to acquire common measurements across species and scales. In the brain, post-mortem MRI facilitates investigations into the origins of image contrast through integration with microscopy1–3, directly addressing concerns over the non-specificity of MRI signals. Long post-mortem scans push the boundaries of spatial resolution for neuroanatomical investigations beyond what is achievable in vivo4–7. Comparative anatomy studies in post-mortem tissue can provide access to species that are not traditionally accessible for in-vivo imaging8–12. As a non-destructive technique, post-mortem MRI facilitates repeat measurements with novel contrasts and technologies.Despite this potential, post-mortem MRI remains a niche approach, in part due to technical challenges and need for multi-disciplinary expertise. Over the past decade, our lab has invested considerable effort in developing novel methodology and acquisition pipelines for post-mortem imaging. Specifically, our research has been aimed at achieving whole-brain post-mortem diffusion MRI to support investigation of multiple brain systems/regions and long-range connections13–15, including in whole, human, post-mortem brains. This investment has led to the acquisition of multiple unique post-mortem datasets, motivating their public release to enable a much broader range of investigations by the imaging community.
In this work, we introduce the Digital Brain Bank, a data release platform providing open access to a number of post-mortem neuroimaging datasets spanning investigations into human neuroanatomy, cross species neuroanatomy, and neuropathology. Datasets provided are the result of over a decade of post-mortem MRI research within our centre. All datasets hosted on the Digital Brain Bank provide post-mortem MRI, including diffusion MRI, with complementary microscopy data (e.g. immunohistochemistry or polarised light imaging [PLI]) included with some datasets. The first release to the Digital Brain Bank contains twenty one distinct whole brain post-mortem MRI datasets, including datasets acquired from whole, human brains.
Datasets
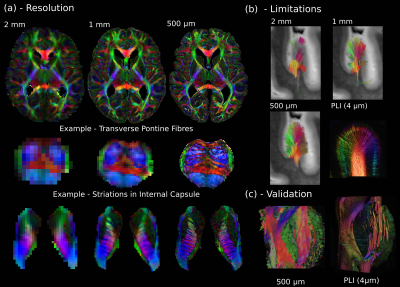
The Digital Brain Bank is accessible at open.win.ox.ac.uk/DigitalBrainBank. Datasets have been organised into categories reflecting three predominant themes of post-mortem neuroimaging research:- Digital Anatomist (Figure 1): Datasets for answering fundamental questions in neuroanatomy, through ultra-high resolution MRI data and complementary microscopy within the same sample.
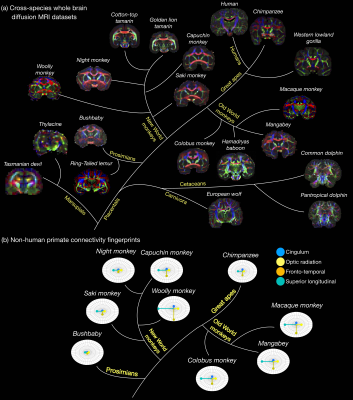
- Digital Brain Zoo (Figure 2): Datasets to investigate neuroanatomy in non-human species and compare anatomy across species.
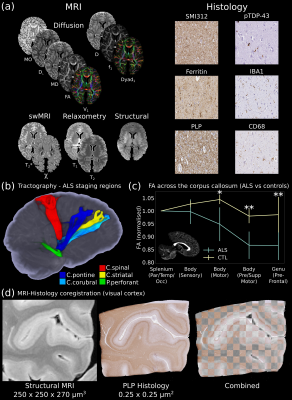
- Digital Pathologist (Figure 3): Datasets for examining neuropathology and MRI-pathology correlates.
The first release to the Digital Brain Bank includes data from multiple published and ongoing projects covering a breadth of neuroimaging research.This includes one of the highest-resolution whole-brain human diffusion MRI datasets ever acquired (500 μm isotropic resolution) alongside complementary PLI (4 μm) (Figure 1)6, whole-brain diffusion MRI in nineteen non-human species (including fourteen non-human primates)16–18 (Figure 2), and one of the largest post-mortem whole-brain cohort imaging studies combining whole-brain multi-modal MRI and microscopy (six histology stains) in human neurodegeneration19, consisting of twelve amyotrophic lateral sclerosis (ALS) and three control brains (Figure 3).
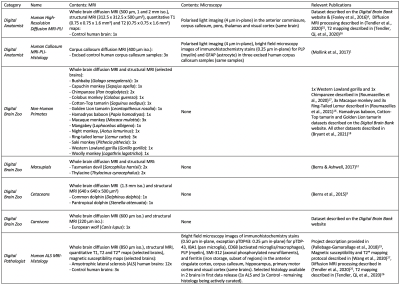
The Digital Brain Bank aims to facilitate the investigation of research hypotheses designed by the user. To reflect this, we primarily provide curated datasets to facilitate these analyses (e.g. whole brain diffusion tensor estimates), as opposed to outputs associated with the results of specific projects (e.g. tractography outputs). A brief description of all the datasets provided with the first data release, alongside relevant publications, is provided in Table 1.
The Digital Brain Bank website has been designed to cover the breadth of spatial scales and modalities encountered in post-mortem imaging. Features on the website facilitate data discovery, with users able to interact with a subset of available datasets directly on the website using Tview: a bespoke, open-source, web-based image viewer. Tview is based on software originally used to display satellite imagery at multiple elevations, enabling real-time visualisation, interaction (zooming/panning), and flexible overlays of different modalities in a single 2D plane of MRI and microscopy data. An example Tview implementation is available at open.win.ox.ac.uk/DigitalBrainBank/#/tileviewer.
Data Access
The Digital Brain Bank aims to minimise the burden on the user to download datasets. All datasets will be available to researchers to use within their own studies. Some datasets are available to directly download on the Digital Brain Bank website. For the majority of datasets, we are developing conditions of use terms via a material transfer agreement (MTA), which users will be asked to agree to prior to access. For datasets restricted by MTAs, when possible a subset of example data (e.g. single subject data) is available to download directly on the website.Conclusion
The Digital Brain Bank is a new data platform providing open access to curated, post-mortem neuroimaging datasets associated with both departmental and collaborative projects. The website has been designed to facilitate data discovery, providing details of available datasets and an interactive image viewer. It is envisioned as a growing resource, with the first data release providing twenty one distinct whole brain post-mortem imaging datasets for users to integrate within their own studies.Acknowledgements
Saad Jbabdi, Rogier B. Mars and Karla L. Miller provided equal contribution
The Digital Brain Bank is supported by the Wellcome Trust (202788/Z/16/Z) and Medical Research Council (MRC, MR/K02213X/1). The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z).
KLM, BCT, AS and JM are supported by funding from the Wellcome Trust (202788/Z/16/Z), RBM is supported by funding from the Biotechnology and Biological Sciences Research Council (BBSRC) UK (BB/N019814/1) and the Netherlands Organization for Scientific Research NWO (452-13-015), SJ is supported by funding from the Wellcome Trust (221933/Z/20/Z, 215573/Z/19/Z) and the MRC (MR/L009013/1), TH and DM are supported by funding from the Wellcome Centre for Integrative Neuroimaging, OA is supported by funding from the Medical Research Council, Alzheimer’s UK and NIHR Oxford Biomedical Research Centre, MFB is supported by funding from the Alfred Benzon’s Foundation, KLB is supported by funding from the Biotechnology and Biological Sciences Research Council (BBSRC) UK (BB/N019814/1), SF and MPG are supported by funding from the MRC (MR/K02213X/1), MPvdH is supported by the Netherlands Organization for Scientific Research NWO (VIDI-452-16-015, ALW-179) and the European Research Council (ERC-COG 101001062), AFDH and IH are supported by funding from the Engineering and Physical Sciences Research Council (EPSRC, EP/L016052/1) and Medical Research Council (MRC, grant MR/L009013/1), AAK was funded by Cancer Research UK (grant C5255/A15935), PRM is supported by funding from the National Research Foundation of South Africa, RALM is supported by funding from the Medical Research Council (MR/K01014X/1) and the Wellcome Trust (202788/Z/16/Z), LR is supported by funding from the Biotechnology and Biological Sciences Research Council (BBSRC) UK (BB/M011224/1), JS is supported by funding from the IDEXLYON IMPULSION 2020 (IDEX/IMP/2020/14) and Labex CORTEX (ANR-11-LABX-0042) grant (Université de Lyon), CS is supported by funding from the NIHR Oxford Biomedical Research Centre (BRC), MRT is supported by funding from the Motor Neurone Disease Association, CW is supported by funding from the China Scholarship Council (CSC).
Human post-mortem brain datasets for the Digital Anatomist and Digital Pathologist used tissue provided by the Oxford Brain Bank, a research ethics committee (REC) approved, HTA regulated research tissue bank (REC reference 15/SC/0639). The Oxford Brain Bank is supported by the MRC, Brains for Dementia Research (BDR) (Alzheimer Society and Alzheimer Research UK), and the NIHR Oxford Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Datasets for the Digital Brain Zoo used tissue provided by the Australian Museum, Copenhagen Zoo, Primate Brain Bank, Save the Tasmanian Devil, Smithsonian, University of Oxford, and Zoological Society of London.
References
1. Mollink J, Kleinnijenhuis M, Cappellen van Walsum AM van, et al. Evaluating fibre orientation dispersion in white matter: Comparison of diffusion MRI, histology and polarized light imaging. Neuroimage. 2017.
2. Keren NI, Taheri S, Vazey EM, et al. Histologic validation of locus coeruleus MRI contrast in post-mortem tissue. Neuroimage. 2015.
3. Langkammer C, Schweser F, Krebs N, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage. 2012.
4. Edlow BL, Mareyam A, Horn A, et al. 7 Tesla MRI of the ex vivo human brain at 100 micron resolution. Sci Data. 2019.
5. Fritz FJ, Sengupta S, Harms RL, Tse DH, Poser BA, Roebroeck A. Ultra-high resolution and multi-shell diffusion MRI of intact ex vivo human brains using kT-dSTEAM at 9.4T. Neuroimage. 2019.
6. Foxley S, Mollink J, Jbabdi S, et al. Validating tractography of high resolution post-mortem human brain at 7T with polarized light imaging. In: ISMRM 24th Annual Meeting Singapore. 2016.
7. Weigel M, Dechent P, Galbusera R, et al. Imaging
multiple sclerosis pathology at 160 μm isotropic resolution by human
whole-brain ex vivo magnetic resonance imaging at 3 T. Sci Rep. 2021.
8. Grewal JS, Gloe T, Hegedus J, et al. Brain gyrification in wild and domestic canids: Has domestication changed the gyrification index in domestic dogs? J Comp Neurol. 2020.
9. Berns GS, Cook PF, Foxley S, Jbabdi S, Miller KL, Marino L. Diffusion tensor imaging of dolphin brains reveals direct auditory pathway to temporal lobe. Proc R Soc B Biol Sci. 2015.
10. Heuer K, Gulban OF, Bazin P-L, et al. Evolution of neocortical folding: A phylogenetic comparative analysis of MRI from 34 primate species. Cortex. 2019.
11. Bhagwandin A, Haagensen M, Manger PR. The brain of the black (Diceros bicornis) and white (Ceratotherium simum) African rhinoceroses: morphology and volumetrics from magnetic resonance imaging. Front Neuroanat. 2017.
12. Berns GS, Ashwell KWS. Reconstruction of the cortical maps of the Tasmanian tiger and comparison to the Tasmanian devil. PLoS One. 2017.
13. Miller KL, McNab JA, Jbabdi S, Douaud G. Diffusion tractography of post-mortem human brains: Optimization and comparison of spin echo and steady-state free precession techniques. Neuroimage. 2012.
14. Miller KL, Stagg CJ, Douaud G, et al. Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage. 2011.
15. Foxley S, Jbabdi S, Clare S, et al. Improving diffusion-weighted imaging of post-mortem human brains: SSFP at 7T. Neuroimage. 2014.
16. Roumazeilles L, Lange FJ, Benn RA, et al. Cortical morphology and white matter tractography of three phylogenetically distant primates: Evidence for a simian elaboration. Cereb Cortex. 2021.
17. Roumazeilles L, Eichert N, Bryant KL, et al. Longitudinal connections and the organization of the temporal cortex in macaques, great apes, and humans. PLoS Biol. 2020.
18. Bryant K, Ardesch DJ, Roumazeilles L, et al. Diffusion MRI data, sulcal anatomy, and tractography for eight species from the Primate Brain Bank. Brain Struct Funct. 2021.
19. Pallebage-Gamarallage M, Foxley S, Menke RAL, et al. Dissecting the pathobiology of altered MRI signal in amyotrophic lateral sclerosis: A post mortem whole brain sampling strategy for the integration of ultra-high-field MRI and quantitative neuropathology. BMC Neurosci. 2018.
20. Schilling K, Gao Y, Janve V, Stepniewska I, Landman BA, Anderson AW. Confirmation of a gyral bias in diffusion MRI fiber tractography. Hum Brain Mapp. 2018.
21. Cottaar M, Bastiani M, Boddu N, et al. Modelling white matter in gyral blades as a continuous vector field. Neuroimage. 2021.
22. Kassubek J, Müller H-P, Del Tredici K, et al. Diffusion tensor imaging analysis of sequential spreading of disease in amyotrophic lateral sclerosis confirms patterns of TDP-43 pathology. Brain. 2014.
23. Hofer S, Frahm J. Topography of the human corpus callosum revisited-Comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006.
24. Huszar IN, Pallebage-Gamarallage M, Foxley S, et al. Tensor Image Registration Library: Automated Non-Linear Registration of Sparsely Sampled Histological Specimens to Post-Mortem MRI of the Whole Human Brain. bioRxiv. 2019.
25. Tendler BC, Foxley S, Hernandez-Fernandez M, et al. Use of multi-flip angle measurements to account for transmit inhomogeneity and non-Gaussian diffusion in DW-SSFP. Neuroimage. 2020.
26. Tendler BC, Qi F, Foxley S, et al. A method to remove the influence of fixative concentration on post-mortem T2 maps using a Kinetic Tensor model. Human Brain Mapping. 2021.
27. Wang C, Foxley S, Ansorge O, et al. Methods for quantitative susceptibility and R2* mapping in whole post-mortem brains at 7T applied to amyotrophic lateral sclerosis. Neuroimage. 2020.
Figures