2394
DTI to Genetics: The Role of HFE H63D gene mutation in White Matter degeneration in APOE4 carrier of Alzheimer’s disease1Departments of Neurosurgery, Pennsylvania State University College of Medicine, PA, United States; Department of Radiology, Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China, Hershey, PA, United States, 2Departments of Radiology, Pennsylvania State University Colle, Hershey, PA, United States, 3Departments of Neurosurgery, Pennsylvania State University College of Medicine, PA, United States, Hershey, PA, United States, 4Departments of 1Neurosurgery and 2Radiology, Pennsylvania State University College of Medicine, PA, United States, Hershey, PA, United States
Synopsis
HFE gene mutation involves iron metabolism, which is shown to directly contribute to myelination process. White matter degeneration in bi-genetic mutation (HFEH63D and ApoE4 allele) carriers was investigated using DTI from the AD Neuroimaging Initiative (ADNI) database. Comparing to the HFEWT/ApoE4+ group, mean diffusivity and radial diffusivity of inferior longitudinal fasciculus in the HFEH63D/ApoE4+ group demonstrated less integrity damage and fewer late-myelination loss with aging reflecting a possible mechanism as HFE polymorphism protective role in partly eliminating ApoE4 being the high-risk factor for AD progressing.
INTRODUCTION
Etiology of AD is related to the iron and lipid imbalances in the brain. The HFE protein is well known to involve in iron homeostasis regulation, and single polymorphisms (SNPs) within the HFE gene sequence (C282Y and H63d) were considered as genetic risks for iron overload genetic disorder and neurodegenerative disorders. Furtherly, the HFE missense mutation-4 (HFEH63D, rs1799945) is involved in alterations in brain iron and cholesterol metabolism in orthologous mice (HFEH67D),2 which is likely involved with the effect of ApoE4 in AD pathophysiology.3 We have delineated HFEH63D is involved in aging brain white matter integrity,4,5 decreasing white matter integrity on MRI metrics in aged cognitively normal people.4 This study investigated its relationship to the AD white matter degeneration in ApoE4 carriers using DTI.METHODS
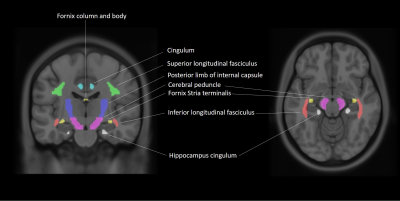
Via Plink software, 40 AD and 78 age- and sex-matched cognitively normal (CN) ApoE4 allele carriers with and without HFEH63D polymorphism were identified (Table 1) from ADNI database (ADNIGO, ADNI2 and ADNI3). (https://ida.loni.usc.edu/pages/access/studyData.jsp?categoryId=11). AD and CN subjects were further divided into four cohorts based on genetic information, including the HFE H63D mutation gene and ApoE4 allele carrier CN cohort (HCH63D/APOE4+) and AD cohort (ADH63D/APOE4+), the HFE wild type and ApoE4 allele carrier CN cohort (HCWT/APOE4+) and AD cohort (ADWT/APOE4+). Cognitive dysfunction was evaluated with MoCA, MMSE, ADAS and CDR. DTI data were acquired at 3 T using single-shell DTI acquisitions. DTI parametric maps (MD, RD and FA) were estimated using DTI-studio and 7 white-matter ROIs from the JHU DTI atlas (Fig 1). Voxels with free water signal were excluded from the ROIs using coregistered FLAIR images. Group-wise ROI based analyses of MD, RD and FA were done with age as covariates and clinical classification (AD/CN) condition of groups to examine the influence of HFE H63D on WM degeneration in AD. Correlation of DTI parameters with cognitive scores for each group were completed with linear regression using SPSS.RESULTS
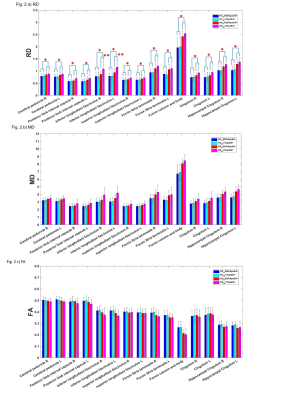
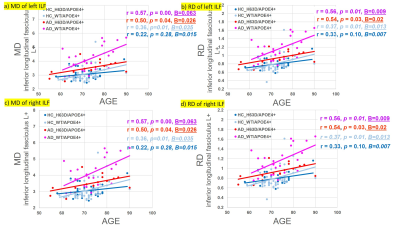
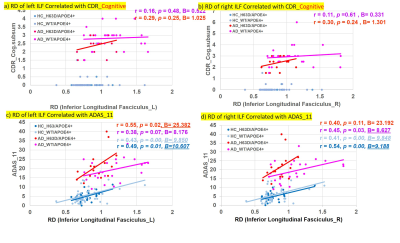
RD, MD and FA demonstrated significantly augmented RD and MD and decreased FA in AD in all the white matter ROIs (ANCOVA with age as a covariate, p<0.05, Figs. 2-4), indicating significant disease-related white matter degeneration. Most interestingly, the degeneration of the white matter appeared less progressed in the AD with H63D mutation. Particularly, RD and MD of AD-H63D subjects were significantly lower than those of AD-WT in bilateral interior longitudinal fasciculus (**, p < 0.05, age as a covariate). Accordingly, the HFEWT/ApoE4+ group exhibited more attenuated CDR sores in cog_subsum than the wild type (Table 1). In general, RD of ILF was positively correlated with ADAS_11 score (p < 0.05) (Fig 4).DISCUSSIONS
The HFEH63D genotype appears to act as an important disease modifier. One possible molecular mechanism is that H63d polymorphism introduces decreased serum hepcidin, which could lead to decreased ferroportin.6,7 It is known that ApoE4+ increases intracellular iron levels of macrophages via lipoprotein oxidation. Conversely, HFEH63D reduces intracellular iron levels of macrophages and its inflammatory response and Aβ aggregation. 3,6,7 Thus, HFEH63D could attenuate the iron dis-homeostasis in macrophages caused by ApoE4, which is a major high-risk factor for AD progression and accelerated aging. Our results, along with recent epidemiology data, showed that HFE polymorphism may offer certain advantages for asymptomatic patients and play protective role in AD neurodegeneration;1,6 considering the potential synergisms with environment and ApoE4 allele.1CONCLUSIONS
DTI analysis demonstrated a consistent trend of WM degeneration in AD with ApoE4 mutation. Such WM degenerations are significantly reduced in the HFEH63D carriers in the AD cohort, most significantly in the bilateral ILF. Cognitively, these AD subjects were also less progressed. Our finding provided evidence that HFE polymorphism plays a protective role in WM degeneration, which, in turn, highlight the importance of iron metabolism in AD pathogenesis.Acknowledgements
We especially thank Center for NMR Research and Center for Aging and Neurodegenerative Diseases of Penn State University College of Medicine for all staffs' kind help and suggestion. This research work is supported by the National Institute on Aging grant (No.1R21AG064486).References
Tisato, V., Zuliani, G., Vigliano, M., et al. Gene-gene interactions among coding genes of iron-homeostasis proteins and APOE-alleles in cognitive impairment diseases. PLoS One, 2018;13(3):1-21. Ali-Rahmani, F., Schengrund C., Connor, J. R., et al. HFE gene variants, iron, and lipids: a novel connection in Alzheimer’s disease. Front. Pharmacol., 2014;5:165. Ayton, S., Faux, N. G., Bush, A. I., et al. Ferritin levels in the cerebrospinal fluid predict Alzheimer's disease outcomes and are regulated by APOE. Nat. Commun., 2015;6(1):6760. 4. Meadowcroft, M. D., Wang, J. L., Purnell, C. J., et al. Reduced Cerebral White Matter Integrity Assessed by DTI in Cognitively Normal H63D-HFE Polymorphism Carriers. J Neuroimaging, 2018;28(1):126-133. 5. Meadowcroft, M. D., Wang, J. L., Purnell, C. J., et al. Reduced white matter MRI transverse relaxation rate in cognitively normal H63D-HFE human carriers and H67D-HFE mice. Brain Imaging Behav, 2016;10(4):1231-1242. Hollerer, I., Bachmann, A., Muckenthaler, M. U. Pathophysiological consequences and benefits of HFE mutations: 20 years of research. Haematologica, 2017;102(5):809-817. Yuan, X. M., Li, W., Baird, S. K., et al. Secretion of ferritin by iron-laden macrophages and influence of lipoproteins. Free Radic Res, 2004;38(10):1133-42.Figures
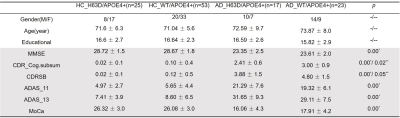
Table 1. Demographics and clinical data for the four cohorts
Note: *, significant difference between HC and AD cohorts (age as covariant); **, significant difference within AD cohorts; -, no significant difference between HC and AD cohorts; --, no significant difference within HC/AD cohorts