2392
Neuroimaging of a novel mouse model of Alzheimer’s Disease: The hAβKI mouse.
Andre Obenaus1, Amandine Jullienne1, Brenda Patricia Noarbe1, Ashley A Keiser2, Michelle Vy Trinh1, Eniko Kramar2, Joy Beardwood2, Tri Dong2, Marcelo Wood2, and MODEL AD3
1Pediatrics, University of California, Irvine, Irvine, CA, United States, 2Neurobiology and Behavior, University of California, Irvine, Irvine, CA, United States, 3University of California, Irvine, Irvine, CA, United States
1Pediatrics, University of California, Irvine, Irvine, CA, United States, 2Neurobiology and Behavior, University of California, Irvine, Irvine, CA, United States, 3University of California, Irvine, Irvine, CA, United States
Synopsis
Mouse models of Alzheimer’s disease (AD) currently do not recapitulate accurately the human disease. The MODEL-AD consortium has recently developed new mouse models of AD, including the human amyloid β knockin (hAβKI) mouse. Using high resolution diffusion MRI (dMRI) we examined regional changes in the hAβKI male and female mouse across its lifespan (4-18mo). Sex and regional differences were apparent with age, including reduced diffusion metrics in the hippocampus, that mirrored altered learning and memory. Preclinical MR phenotyping allows for cross-species comparisons for biomarker identification.
Introduction
The Model Organism Development and Evaluation for Late-Onset Alzheimer’s Disease (MODEL-AD) Consortium was established to develop the next generation of Alzheimer’s disease (AD) models based on human genomic and imaging data. Recently, MODEL-AD has generated a new human Aβ Knock-In (hAβKI) mouse that substitutes 3-amino acids in the mouse Aβ locus with that of the human amyloid precursor protein (App) gene (Baglietto-Vargas et al., 2021). This substitution leads to age-dependent cognitive and synaptic impairments as these mice progressively age. Neuroimaging of AD is a critical component of human diagnoses and preclinical magnetic resonance imaging (MRI) can investigate phenotypic alterations in these new mice models. Specifically, we used diffusion MRI (dMRI) to investigate brain-wide structural alterations in conjunction with connectomic analyses in the hAβKI mouse across its lifespan.Methods
We examined hippocampal synaptic plasticity in slices from male and female hAβKI mice at 4, 12 and 18mo of age (n=5/group/sex/genotype). For imaging a total of 105 male and female hAβKI mice at the same time points (WT n=51, hAβKI n=54; ~n=8/sex/time point). All imaging was performed on a 9.4T (Bruker Biospin, PV5.1) MR scanner. The following sequences were utilized: 1) diffusion tensor imaging (DTI): 5 B0, 30 directions b=0, 3000 mm2/sec, 25 slices 0.5mm thick for full brain coverage, 2) multiecho T2 for quantitative relaxation, 3) 3D T2 RARE for volumetric assessments and 4) susceptibility weighted imaging for iron deposition. Our automated pipeline for regional analyses utilized the Australian Mouse Brain Mapping Consortium (AMBMC) atlas which we split for bilateral measurements. DTI metrics (axial (AxD), radial (RD), mean (MD) diffusivities and fractional anisotropic (FA) maps) data were extracted from 42 bilateral regions encompassing cortical, subcortical and white matter regions. Tractography and connectomics were derived using DSI studio.Results
Impairments in hippocampal long-term (LTP) were observed in male hAβKI mice at 4 months of age relative to WT with no significant change observed in females. In female hAβKI mice, impairments in LTP were observed at 12 and 18 months of age relative to WT with no significant change observed in males (Fig. 1A). Brain volumes were significantly decreased in male hAβKI compared to WT mice, but no changes were found in female hAβKI mice. Volumetric increases in select brain regions, such as the anterior cingulate cortex in male hAβKI mice compared to WT were observed but was not related to tissue relaxation (T2) (Fig. 1). dMRI heatmaps of MD highlighted regional differences between males and females across time and genotype, with MD progressively decreasing with time (Fig. 2). Statistical analyses for regional changes showed that male hAβKI mice had progressive changes within the brain with advancing age whereas the largest dMRI changes in females was at 12mo of age (Fig. 3). For example, AxD in 18mo male hAβKI mice was increased in 4 of 7 white matter regions but not in female hAβKI mice compared to WT. This would suggest regional and temporal sensitivity to diffusion metrics in the hAβKI mouse brain. As these animals exhibit impaired hippocampal long-term potentiation, we probed the CA1 region for diffusion abnormalities. Compared to male WT mice, we found decreased RD and MD but no change in AxD whereas FA was increased within the CA1 region of the hippocampus of male hAβKI mice (no change in female mice). Ongoing multishell dMRI analyses will report neurite and cellular density measures from NODDI modeling.Discussion
Human AD is a complex, progressive and multi-faceted disease process in late-onset AD (LOAD) patients. At the present time there are no animal models that recapitulate all the known features in LOAD patients. The MODEL-AD consortium has been tasked to develop better LOAD mouse models. One such recently developed model is the hAβKI mouse. In male hAβKI mice dMRI metrics, particularly RD and MD (along with FA) appeared to be sensitive to identify ongoing histopathological perturbations. The hippocampus of male hAβKI mice but not females, showed reduced diffusion (RD, MD) consistent with altered tissue structure. White matter was also similarly affected but to an even larger extent. These findings complement our observations of impaired hippocampus-dependent memory and LTP in hAβKI mice.Conclusion
Preclinical MRI can be used to phenotype new mouse models of AD. Using a newly developed mouse model of AD, the hAβKI mouse, we identified progressive altered regional tissue metrics. The hippocampal CA1 region in the hAβKI mouse appeared to be particularly vulnerable, findings consistent with observed impairments in confirmed LTP and behavioral findings. MRI imaging of new mouse models is a powerful tool to investigate tissue level modifications to brain structure and function. Comparison of MRI from mouse models of AD and those from human MRI acquisitions provides the opportunity to compare and contrast species for evaluation of potential imaging biomarkers.Acknowledgements
MODEL-AD was established with funding from The National Institute on Aging (U54 AG054345-01, U54 AG054349-01).References
Baglietto-Vargas, D., Forner, S., Cai, L. et al. Generation of a humanized Aβ expressing mouse demonstrating aspects of Alzheimer’s disease-like pathology. Nat Commun 12, 2421 (2021). https://doi.org/10.1038/s41467-021-22624-zFigures
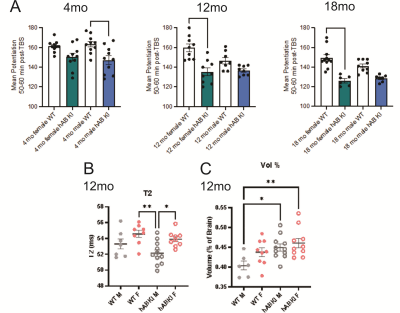
Fig. 1
Long-term potentiation (LTP) and MRI of hAβKI mice: A) LTP was impaired in hAβKI male mice at 4 mo of age relative to
WT with no significant change observed in females. At 12 and 18 mo, LTP was
impaired in hAβKI
female mice relative to WT with no changes observed in males. B) T2 relaxation
in the anterior cingulate cortex (ACC) was not significantly different between
genotype but hAβKI
male mice had significantly lower T2 values than female WT and hAβKI mice. C) At 12mo of age, WT males had
lower ACC volumes than male and female hAβKI
mice. *p<0.05, ***p<0.001, ****p<0.0001.
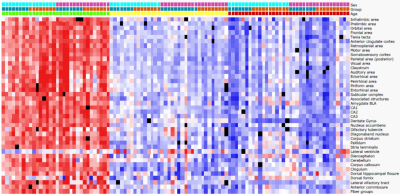
Fig. 2. Heatmap of regional mean diffusivity (MD) from 4-18mo old by sex
and genotype. Clear differences can be observed as hAβKI mice age compared to
WT mice, with progressive reductions in MD. Similar patterns were observed in
RD and AxD.
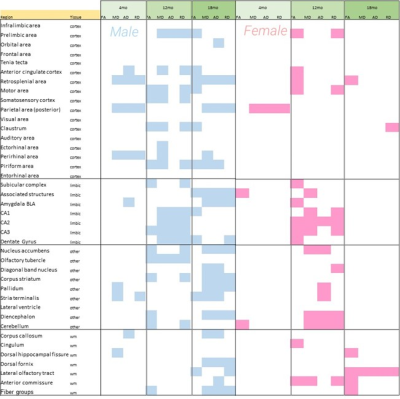
Fig. 3. Significant regional changes in male and female hAβKI mice relative
to WT mice are plotted. Male hAβKI mice have progressive regional changes in
most dMRI metrics up to 18mo of age, whereas female mice exhibit increased
regional changes only at 12mo and only white matter changes at 18mo. This
suggests regional and sex vulnerabilities in the hAβKI mouse.
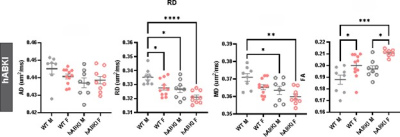
Fig. 4.
Hippocampal CA1 dMRI metrics are reduced in hABKI mice at 12mo of age. RD and
MD had significant reductions within the CA1 region compared to WT mice that
were associated with concomitant increases in FA. Male hAβKI mice, but not
female mice had the most significant changes in the CA1.
DOI: https://doi.org/10.58530/2022/2392