2391
Automatic detection of the anterior choroidal artery in TOF-MRA and its trajectory through the hippocampus and its sub-regions1Faculté de Médecine et des Sciences de la Santé, Université de Sherbrooke, Sherbrooke, QC, Canada, 2Médecine nucléaire et radiobiologie, Université de Sherbrooke, Sherbrooke, QC, Canada, 3Anesthésiologie, Université de Sherbrooke, Sherbrooke, QC, Canada, 4Service de Neurologie, Département de Médecine, CHUS, Sherbrooke, QC, Canada, 5Clinique de la Mémoire et centre de recherche sur le Viellissement, CIUSSS de l'Estrie-CHUS, Sherbrooke, QC, Canada
Synopsis
The Anterior Choroidal Artery (AChA) is a small artery in the cerebral vasculature that perfuses the hippocampus. Its role in brain pathologies and cognitive decline is poorly understood. This could be due to the fact that there is no current standard method to analyse this artery in Time-of-Flight Magnetic Resonance Angiography (TOF-MRA). Here, we propose a fully automated approach for accurately localizing and quantifying the AChA in young healthy subjects. We then used this method to analyse the trajectory of the AChA through the hippocampus and its sub-regions.
Introduction
The Anterior Choroidal Artery (AChA) is a small bilateral artery stemming from the Circle of Willis, as a bifurcation of the internal carotid arteries1. This artery perfuses several important subcortical regions of the brain including the hippocampus2,3. The AChA is of clinical importance due to its involvement in several pathologies of the brain. For example, up to 11% of strokes are believed to originate from the AChA4. Additionally, the AChA may have a role in the pathophysiology of Parkinson's disease2. Previous research has shown that abnormal size and shape of cerebral vascularisation is associated with a greater risk of cognitive impairment, which itself is associated with stroke and Parkinson’s disease5,6. Currently, there is a lack of tools available to researchers who wish to study the morphology of the AChA from Time-of-Flight Magnetic Resonance Angiography (TOF-MRA). Here, we present a fully automated approach using a convolutional neural network to accurately localize and label the AChA. Moreover, we applied this method to assess the trajectory of AChA in the hippocampus and its sub-regions.Methods
To design an AChA automatic segmentation tool, we used a Convolutional Neural Network (CNN) that can perform multilabel segmentation of the Circle of Willis developed in our laboratory7. We then added the AChA to this approach by training it on 232 manual annotations of the AChA all performed by a medical expert. Figure 1 shows an example of an AChA annotation and a CNN prediction. The quality of the AChA predictions described above was evaluated by a qualitative and quantitative assessment. The qualitative evaluation was performed on 59 brains of the OASIS dataset8. Here, the predicted location of the AChA was assigned a correct or incorrect rating. To get a correct rating, the CNN prediction was compared to the raw TOF and had to visually overlap the AChA correctly. The quantitative evaluation was performed using the Dice Score (DS)9 to give a measure of similarity and the Interclass Correlation Coefficient (ICC) to measure the variability. First, DS was computed on the similarity between the predictions on a test set of 15 participants and their corresponding manual annotations. The ICC was computed using a publicly available dataset of 10 subjects, each scanned 4 times10.Additionally, 20 healthy participants were imaged on a 3T Ingenia scanner equipped with a 32-channel head-coil (Philips Healthcare, Best, Netherlands). First, a 3D gradient-echo T1 weighted image was collected (field of view (FOV)=240X240X161 mm; TR/TE=7.9/3.5ms, 1 mm isotropic voxels, flip angle(FA)= 8°), the T1-image was processed with FastSurfer11 and VolBrain12 to segment and compute the volume of the hippocampus and three of its sub-regions (Cornu Ammonis (CA) 1-3, subiculum, and CA4/Dentate Gyrus (CA4/DG)). Next, a high resolution, whole-brain multi-band TOF image was collected (FOV= 200X200X120.9mm, TR/TE= 23/3.45ms, FA= 18°, parallel imaging (SENSE) acceleration factor=3, acquisition resolution of 0.65X0.65X1.30, reconstructed resolution of 0.626x0.625x0.65mm). From this, the CNN-segmented AChA and the hippocampus segmentations were registered in MNI 152 space13 using ANTs14. To look at the trajectory of the AChA in the hippocampus, we computed the intersection of the AChA and the hippocampus in all subjects. Next, we averaged the region of intersection across the 20 participants to generate a probability map of where the AChA overlapped the hippocampus. Lastly, we computed the shortest distance between AChA, the hippocampus and its subdivisions.
Results
The results of the qualitative and quantitative evaluation are shown in figure 2. The qualitative evaluation demonstrated 86% accuracy in the AChA prediction, the DS indicated a 58% ± 17% accuracy and the ICC was 0.72-0.9. Figure 3 shows an example of the AChA with respect to the hippocampus and its sub-regions. We found that the average distance between the AChA and the hippocampus, the CA1-3, the subiculum and the CA4/DG were 0.1mm ± 0.5, 0.7mm ± 1.1, 5.2mm ± 2.5, and 7.6mm ± 7.4, respectively (figure 4). These four distances were statistically different from each other, which implies that AChA has a trajectory in the hippocampus which is closer to the CA1-3 than to the subiculum or the CA4/DG. As demonstrated in figure 5, AChA intersected the anterior portion of the hippocampus. More precisely, the MNI coordinates having the highest overlap between the 20 participants for the intersection between the left AChA and the left hippocampus were x = 76, y = 119, z = 65. For the right hemisphere, the MNI coordinates were x = 116, y = 120, z = 66.Discussion & Conclusion
Here we propose an approach that can automatically and accurately locate and quantify the AChA in TOF-MRA images. Our results confirm that AChA perfuses the hippocampus2,3. In addition, we have shown that the AChA intersects the hippocampus in its anterior portion with the CA1-3 being closest to this artery. As this region is important for cognitive functions and episodic memory15, understanding morphological differences of the AChA across individuals may advance our understanding of how deficits in these processes develop. Thus, morphological variations of the AChA may have an important role in the pathophysiology of cognitive impairment and warrants further study.Acknowledgements
No acknowledgement found.References
1. Blumenfeld H. Neuroanatomy through clinical cases. (Stinauer Associates, 2010).
2. Yu J, Xu N, Zhao Y, Yu J. Clinical importance of the anterior choroidal artery: a review of the literature. Int J Med Sci. 2018;15(4):368. doi:10.7150/IJMS.22631
3. Perosa V, Priester A, Ziegler G, et al. Hippocampal vascular reserve associated with cognitive performance and hippocampal volume. Brain. 2020;143(2):622-634. doi:10.1093/BRAIN/AWZ383
4. Hupperts RMM, Lodder J, Heuts-van Raak EPM, Kessels F. Infarcts in the anterior choroidal artery territoryAnatomical distribution, clinical syndromes, presumed pathogenesis and early outcome. Brain. 1994;117(4):825-834. doi:10.1093/BRAIN/117.4.825
5. Gutierrez J, Guzman V, Xasiyev F, et al. Brain arterial dilatation and the risk of Alzheimer Disease. Alzheimers Dement. 2019;15(5):666. doi:10.1016/J.JALZ.2018.12.018
6. Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 2016 71. 2016;7(1):1-14. doi:10.1038/ncomms11934
7. Dumais F, Caceres MP, Bocti NA-BC, Whittingstall K. Automatic multilabel segmentation of large cerebral vessels from MR angiography images using deep learning. In: ISMRM; 2021.
8. LaMontagne PJ, Benzinger TLS, Morris JC, et al. OASIS-3: Longitudinal Neuroimaging, Clinical, and Cognitive Dataset for Normal Aging and Alzheimer Disease. medRxiv. Published online December 15, 2019:2019.12.13.19014902. doi:10.1101/2019.12.13.19014902
9. Dice LR. Measures of the Amount of Ecologic Association Between Species. Ecology. 1945;26(3):297-302. doi:10.2307/1932409
10. Midnight scan club, https://www.cell.com/neuron/pdf/S0896-6273(17)30613-X.pdf.
11. Henschel L, Conjeti S, Estrada S, Diers K, Fischl B, Reuter M. FastSurfer - A fast and accurate deep learning based neuroimaging pipeline. Neuroimage. 2020;219:117012. doi:10.1016/J.NEUROIMAGE.2020.117012
12. Manjón J V., Coupé P. Volbrain: An online MRI brain volumetry system. Front Neuroinform. 2016;10(JUL):30. doi:10.3389/FNINF.2016.00030/BIBTEX
13. Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54(1):313-327. doi:10.1016/J.NEUROIMAGE.2010.07.033
14. Avants BB, Tustison NJ, Stauffer M, Song G, Wu B, Gee JC. The Insight ToolKit image registration framework. Front Neuroinform. 2014;8(APR). doi:10.3389/FNINF.2014.00044
15. Zeidman P, Maguire EA. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci. 2016;17(3):173. doi:10.1038/NRN.2015.24
Figures
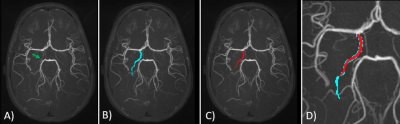
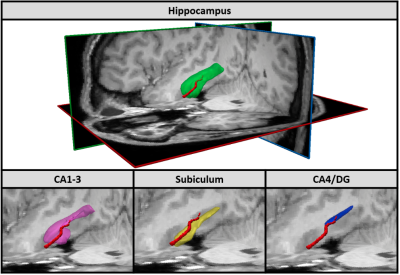
Figure 3: In the upper part of the figure, left antero-superior view of the right Anterior Choroidal Artery (AChA) (red) and right hippocampus (green). In the lower part of the figure, left antero-superior view of the right AChA and three sub-regions of the hippocampus: CA1-3 (pink), subiculum (yellow) and CA4/DG (blue).
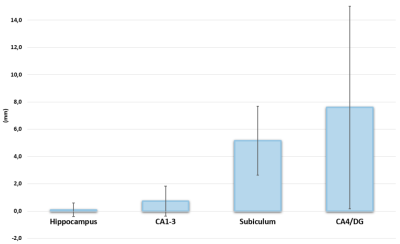
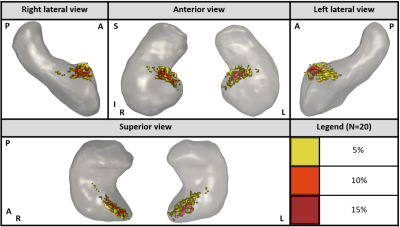
Figure 5: Several 3D views of the hippocampus (gray) with Anterior Choroidal Artery (AChA) intersection regions in color. The different shades of warm colors represent the percentage of the 20 healthy participants having this region in common. This figure demonstrates that the AChA intersects the hippocampus in its anterior portion. (R: Right / L: Left / A: Anterior / P: Posterior / S: Superior / I: Inferior)