2387
Neurovascular dysfunction in patients with subjective cognitive decline: A resting-state fMRI study1Radiology department, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China, 2Rehabilitation Department, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China, 3Rehabilitation Department, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, China, 4MR Research China, GE Healthcare, Beijing, China
Synopsis
The main goal of this study was to investigate the changes of neurovascular coupling (NVC) in patients at early stages of Alzheimer’s disease. Patients with SCD and MCI were enrolled. Significantly changes of neurovascular coupling have been separately found between SCD, MCI patients and HCs in the regions involved the left Rolandic operculum, left anterior orbital gyrus, right insula, right parahippocampal gyrus, left lingual gyrus, median cingulate and paracingulate gyri and left supramarginal gyrus. In addition, these changes of NVC showed strong correlations with multiple clinical scales. Therefore, NVC can be an effective method for early diagnosis of SCD patients.
Introduction
Alzheimer's Disease (AD), as the most common form of dementia, has a continuous pathological process, including AD-subjective cognitive decline (SCD), AD-mild cognitive impairment (MCI) and AD-dementia stages[1].Neurovascular coupling (NVC) is a newly defined term, representing a relationship that the neurovascular unit facilitates cerebral blood flow (CBF) changes in response to the metabolic activity of neurons [2]. Dysfunction of NVC can cause further cerebral pathologies and neurological disorders. So far, neurovascular uncoupling has been increasingly reported in patients with AD, suggesting neurovascular dysregulation in AD pathogenesis[3, 4]. However, the NVC in SCD patients remains unknown, and the relationship of NVC with cognitive assessments is also not clear.
Therefore, this study mainly aimed to firstly investigate the NVC in SCD and MCI patients as well as healthy controls (HC)s, and to secondly explore the relationship of the corresponding NVC with clinical scales.
Materials and Methods
Subjects31 SCD patients (mean: 67.97±6.80 years), 33 MCI patients (mean: 71.27±6.97 years) and 27 well-matched HCs (mean: 70.92±6.75 years) were recruited in this study. Each subject was also assessed with multiple clinical scales, including overall cognitive function assessed by Mini-Mental-State-Examination (MMSE), Montreal-Cognitive-Assessment (MoCA); speed or executive function assessed by Trail-Making-Test (TMT) A&B and Symbol digit modalities test (SDMT); language function assessed by Boston-Naming-Test (BNT); episodic memory assessed by auditory-verbal learning test-Huashan version (AVLT-H); memory function assessed by Webster Logical Memory Test (WLMT). Written consent was obtained from each patient.
MRI experiment
All MR experiments were performed at a 3T-MR scanner (750W, GE, USA) equipped with a 24-channel head coil.
High-resolution T1 weighted MR images were acquired with BRAVO sequence. In fMRI measurement, multi-phase echo-planar-imaging (EPI) sequence sensitive to blood oxygenation level dependent (BOLD) contrast was applied. For cerebral perfusion measurement, a fast-spin-echo based 3D ASL technique was used. The corresponding scan parameters were shown in Table1. Total scan time was less than 20 minutes.
Data analysis
All pre-processing procedures were performed using the Data Processing & Analysis for Brain Imaging (DPABI_V5.0 http://rfmri.org/dpabi) based on SPM12, including the removal of the first 10 time points, slice timing, realign, normalization, detrend and covariance regression.
ALFF was used to represent as activity of neurons and it was defined by the averaged square root of the power spectrum via fast Fourier transform (FFT) across a 0.01–0.08 Hz window at each voxel, Z-score transformed and smoothed after preprocessing procedures. In this study, patients were excluded with head motions exceeding 2mm of translation or 2 degrees of rotation. CBF images were obtained using a vendor-provided ASL post-processing software in ADW4.6 workstation (GE Healthcare). The processing procedures of CBF maps included normalization, Z-score transformation and smoothing. 94 independent regions of cerebrum were segregated using the automated anatomical labeling (AAL) 2 atlas and fused separately on CBF and ALFF maps. The correlation coefficient between neuronal activity and cerebral perfusion, defined as NVC, was calculated for each brain sub-region.
All statistical analyzes were performed in SPSS software (23.0). One-way analysis-of-variance (ANOVA) with the factor of group and subsequent post-hoc-t tests were applied to compare NVC differences among SCD, MCl patients and HCs (FDR correction, p value<0.05). In addition, Pearson correlation analysis was performed to test the relationship between each clinical scale score and NVC. Significance threshold was set as p<0.05.
Results
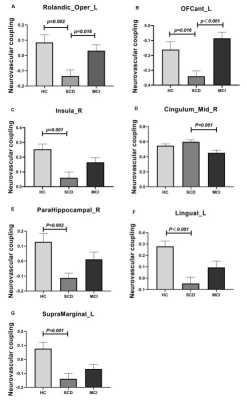
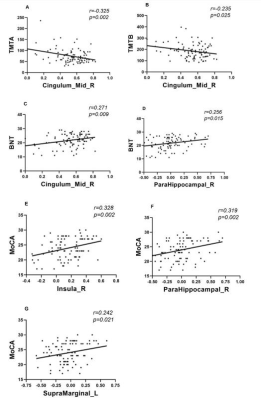
ANOVA analysis revealed significant different NVC between patients with MCI and SCD and HC groups in the left Rolandic operculum(Rolandic_Oper_L), left anterior orbital gyrus(OFCant_L), right insula(Insula_R), right parahippocampal gyrus(ParaHippocampal_R), left lingual gyrus(Lingual_L), median cingulate and paracingulate gyri(Cingulum_Mid_R) and left supramarginal gyrus(SupraMarginal_L). The corresponding post-hoc-t test further indicated that compared with HC group (Fig.1), SCD patients showed lower NVC in the Rolandic_Oper_L, OFCant_L, Insula_R, ParaHippocampal_R, Lingual_L and SupraMarginal_L. NVC in MCI patients were found to be higher than SCD patients in the Rolandic_Oper_L and OFCant_L. Lower NVC at the Cingulum_Mid_R were also found in MCI than SCD group.NVC in Cingulum_Mid_R was negatively correlated with TMT-A(r=-0.325, p=0.002) and TMT-B(r=-0.235, p=0.025). The BNT was positively correlated with NVC in Cingulum_Mid_R(r=0.271, p=0.009) and ParaHippocampal_R(r=0.256, p=0.015). Meanwhile, MoCA showed a significant positive correlation with NVC in Insula_R(r=0.328, p=0.002), ParaHippocampal_R(r=0.319, p=0.002) and SupraMarginal_L(r=0.242, p=0.021)(Fig.2).
Discussion and conclusion
In this study, SCD patients showed significant disruption of blood supply-neural activity coupling. For MCI patients, the increased NVC in Rolandic_Oper_L and OFCant_L might be interpreted as a compensatory mechanism. Parahippocampal gyrus is associated with episodic memory[5]. The cingulate gyrus is the main component of limbic system and also participates in the composition of Papez circuit[6]. Its main functions include sensation, motor regulation and cognition. Supramarginal gyrus is related to language perception and processing. Additionally, regional changes in neurovascular coupling were correlated with clinical scales. The strong association of neurovascular coupling with clinical severity indicate the significant involvement of vascular dysfunction in AD pathogenesis.In conclusion, neurovascular coupling might be considered an effective biomarker for the detection of neurovascular dysfunction in early stage of AD.
Acknowledgements
No acknowledgement found.References
[1] Jack C R, Jr., Bennett D A, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease [J]. Alzheimers Dement, 2018, 14(4): 535-562.
[2] Pasley B, Freeman R. Neurovascular coupling [J]. Scholarpedia, 2008, 3(3): 5340.
[3] Kisler K, Nelson A R, Montagne A, et al. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease [J]. Nature Reviews Neuroscience, 2017, 18(7): 419-434.
[4] Tarantini S, Tran C H T, Gordon G R, et al. Impaired neurovascular coupling in aging and Alzheimer's disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline [J]. Exp Gerontol, 2017, 94: 52-58.
[5] Düzel E, Habib R, Rotte M, et al. Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configurations [J]. J Neurosci, 2003, 23(28): 9439-9444.
[6] Vogt B A, Finch D M, Olson C R. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions [J]. Cereb Cortex, 1992, 2(6): 435-443.
Figures
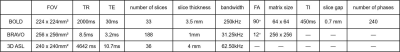
TR=repetition time; TE=echo time; FA=flip angle; TI=inversion time.