2382
Resting-State Functional Connectivity of the Central Autonomic Network (CAN) in Parkinson’s Disease1Diagnostic Radiology, Singapore General Hospital, Singapore, Singapore, 2Graduate Institute of Medicine, Yuan Ze University, Taipei, Taiwan, 3Neurology, National Neuroscience Institute, Singapore, Singapore
Synopsis
Vast evidence for network-level functional dysfunctions have been reported in Parkinson’s disease (PD). However, brain abnormalities that underlie common autonomic symptoms in PD have not been fully investigated. Resting-state functional integration of the Central Autonomic Network (CAN) of 79 PD patients and 43 healthy controls (HC) were evaluated using seed-based analysis. Significantly decreased functional connectivity between the right anterior insular seed and left lateral occipital cortex, right lingual gyrus, and left occipital pole were found in HC compared to PD. Functional connectivity analysis of the CAN facilitates further understanding of the potential mechanisms underlying altered autonomic regulation in PD.
Introduction
Loss of dopaminergic neurons within the substantia nigra and aggregation of alpha-synuclein deposits as Lewy bodies in neurons are pathological hallmarks of Parkinson’s Disease (PD) 1,2. These result in prominent motor disorders, accompanied by autonomic symptoms. The latter include orthostatic hypotension, swallowing difficulties, sweating, constipation, diarrhea, and urinary storage symptoms, including frequency, urgency, and nocturia3. Resting-state functional magnetic resonance imaging (rs-fMRI) has gained popularity as a non-invasive tool to explore the alterations of spontaneous brain activities4. Increasing evidence for functional alterations of the executive control, dorsal attention, auditory, frontoparietal and frontotemporal networks, including the insular cortex, have been identified in neurodegenerative diseases5-7. However, brain functional abnormalities that underlie the common autonomic symptoms in PD are wanting8. The central autonomic network (CAN) has a critical role in the regulation of the control of body visceral functions, maintenance of homeostasis, and adaptation to internal or external challenges9,10. The four regulatory regions of the CAN, namely posterior midcingulate cortex (pMCC), left amygdala (AMYG), right anterior insula (aINS), and left posterior insula (pINS), are known to be involved in autonomic regulation across cognitive, affective, and somatosensory-motor tasks10. Impaired complex central network which modulates parasympathetic outflow in the resting state has also been reported in the early clinical stages of PD11. We explored the resting-state functional connectivity of CAN using seed-based analysis using the aforementioned four seed regions in a case-control cohort of PD patients and healthy controls.Method
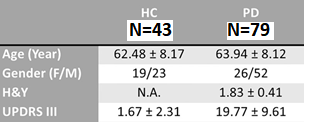
Seventy-nine PD patients and 43 asymptomatic healthy controls (HC) subjects underwent brain MRI on a 3T scanner (Skyra, Siemens Healthcare, Erlangen, Germany) using a 32-channel head coil. The rs-fMRI scan was a 2D multi-slice gradient echo planar imaging sequence with the following parameters: TR/TE = 3000/30ms, FA = 90°, in-plane resolution = 2.13 x 2.13 x 3.3 mm3, number of slices = 44, number of measurements = 150. All subjects were instructed to rest quietly, keeping their eyes open, not to fall asleep, keep their minds relaxed and not think of anything in particular. Clinical assessments included the Hoehn and Yahr staging [H&Y] and Unified Parkinson’s Disease Rating Scale part 3 [UPDRS-III]) for all subjects. Data preprocessing and rs-fmri analysis were performed using CONN functional connectivity toolbox12. The first five measurements of rs-fMRI data were first excluded. Quality control for head motions was conducted. Subjects with averaged framewise displacement of rsfMRI BOLD signals larger than 0.5 mm were excluded. Standard preprocessing pipeline included slice timing correction, motion correction, image co-registration between the rs-fMRI and structural T1-weighted images, T1W segmentation, normalization onto MNI152 template, and spatial smoothing using a 6-mm FWHM. This was followed by nuisance regression using the regressors such as linear detrending, head motion of six rigid body parameters, framewise displacements, and averaged signals from white matter and cerebrospinal fluid. Finally, rsf-MRI data was filtered using a bandpass filter of 0.008–0.09 Hz. Seed-based voxel-wise correlation analysis was performed using seed regions defined at the posterior middle cingulate cortex (pMCC), left posterior insular (pINS), right anterior insular (aINS), and left amygdala gyrus (AMYG)9.Results
The study demographics are found in Table 1. Significant differences in functional connectivity between the right aINS seed and left lateral occipital cortex, right lingual gyrus, and left occipital pole were found between the two groups (Figure 1). Decreased functional connectivity was seen in HC compared to PD after correcting for multiple comparisons (p<0.05 corrected by FDR), using threshold free cluster enhancements and controlling for age and gender. There were no significant findings in the other seed regions. Further correlations were performed between functional connectivity of the right aINS seed with motor scores (H&Y and UPDRS III) but no significant results were found.Discussion
We explored functional alterations of the CAN in PD patients and HC using seed-based rs-fMRI analysis. Significant decrease in regional functional connectivity was seen between the right aINS seed and left lateral occipital cortex, right lingual gyrus, and left occipital pole in control compared to PD. The insula is regarded as the ‘visceral sensory’ cortex and is a somatotopically organised region that receives visceral sensory information and modulates autonomic nervous system responses11. Morphological and fMRI studies have suggested that the ventral aINS, dorsal aINS and the pINS are involved in several brain functions, such as cognition, affect processing, chemosensory function, sensorimotor and interoception of physiological processing13. Several studies have related atrophic gray matter volume and decreased functional connectivity in insula to non-motor symptoms and cognitive impairment in PD patients4,14,15. The altered functional connectivity observed in the CAN in patients with PD could represent either a compensatory attempt to maintain homeostasis, or an a priori pathologic functional activity, or both11.Conclusion
The alteration of functional connectivity during resting-state seen in the CAN network in PD suggests a compensatory attempt to maintain homeostasis, or an a priori pathologic functional activity. Functional connectivity analysis provides new insights on the interaction among global coordination of brain activity in PD.Acknowledgements
We express our appreciation to team of MR Radiographers and research assistants in the Department of Diagnostic Radiology, Singapore GeneralHospital for their kind assistance and excellent support in this study.References
1. Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the "common" neurologic disorders? Neurology. 2007;68(5):326-337.
2. Roy HA, Green AL. The Central Autonomic Network and Regulation of Bladder Function. Frontiers in Neuroscience. 2019;13(535).
3. Jain S. Multi-organ autonomic dysfunction in Parkinson disease. Parkinsonism Relat Disord. 2011;17(2):77-83.
4. Li M, Liu Y, Chen H, et al. Altered Global Synchronizations in Patients With Parkinson's Disease: A Resting-State fMRI Study. Frontiers in aging neuroscience. 2019;11:139.
5. Hafkemeijer A, Möller C, Dopper EG, et al. Resting state functional connectivity differences between behavioral variant frontotemporal dementia and Alzheimer's disease. Front Hum Neurosci. 2015;9:474.
6. Farb NA, Grady CL, Strother S, et al. Abnormal network connectivity in frontotemporal dementia: evidence for prefrontal isolation. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49(7):1856-1873.
7. Sedeño L, Couto B, García-Cordero I, et al. Brain Network Organization and Social Executive Performance in Frontotemporal Dementia. Journal of the International Neuropsychological Society : JINS. 2016;22(2):250-262.
8. Multani N, Taghdiri F, Anor CJ, et al. Association Between Social Cognition Changes and Resting State Functional Connectivity in Frontotemporal Dementia, Alzheimer’s Disease, Parkinson’s Disease, and Healthy Controls. Frontiers in Neuroscience. 2019;13(1259).
9. Sie JH, Chen YH, Chang CY, Yen NS, Chu WC, Shiau YH. Altered Central Autonomic Network in Baseball Players: A Resting-state fMRI Study. Sci Rep. 2019;9(1):110.
10. Beissner F, Meissner K, Bär KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(25):10503-10511.
11. Tessa C, Toschi N, Orsolini S, et al. Central modulation of parasympathetic outflow is impaired in de novo Parkinson's disease patients. PLoS One. 2019;14(1):e0210324.
12. Whitfield-Gabrieli S, Nieto-Castanon A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity. 2012;2(3):125-141.
13. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis - brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49(2):437-445.
14. Pan PL, Song W, Shang HF. Voxel-wise meta-analysis of gray matter abnormalities in idiopathic Parkinson's disease. European journal of neurology. 2012;19(2):199-206.
15. Christopher L, Marras C, Duff-Canning S, et al. Combined insular and striatal dopamine dysfunction are associated with executive deficits in Parkinson's disease with mild cognitive impairment. Brain. 2014;137(Pt 2):565-575.