2380
Regional patterns of nigral degeneration in the substantia nigra in atypical Parkinsonism using neuromelanin-sensitive MRI1PITIE SALPETRIERE, PARIS, France, 2PARIS BRAIN INSTITUTE, PARIS, France, 3Paris Brain Institute, PARIS, France, 4Università degli Studi "G. d'Annunzio" Chieti, CHIETI, Italy
Synopsis
We investigated the regional selectivity of neurodegenerative changes in the SNc in patients with Parkinson’s disease (PD) and atypical parkinsonism using neuromelanin-sensitive MRI. We confirmed that neuromelanin volume and signal were reduced in parkinsonian disorders. The spatial pattern of changes differed between progressive supranuclear palsy (PSP) and synucleinopathies. Compared to healthy controls (HC), subjects with PD and multisystem atrophy (MSA), PSP subjects had greater changes in the associative region. Signal-to-noise in the sensorimotor territory was preserved in PSP, but reduced in PD patients and there was a trend in MSA patients. There was no significant difference between MSA and PD.
INTRODUCTION
Introduction. The hallmark of neurodegenerative parkinsonism is the progressive neurodegeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc).1–3 Degeneration of dopaminergic neurons has been studied in PD and atypical parkinsonism using neuromelanin-sensitive MRI. In Parkinson’s disease, the greatest depletion of dopaminergic neurons was shown to occur first in the posterolateral part of the SNc before spreading to other nigrosomes.4 This pattern has been recently confirmed using neuromelanin-sensitive MRI.5 Regional patterns of nigral neurodegeneration may be different in atypical parkinsonism. Our aim was to investigate the topography of neurodegenerative SNc involvement in patients with PD and atypical parkinsonian syndromes in comparison with healthy controls (HC) using neuromelanin-sensitive MRI.METHODS
Methods. Twenty-two healthy controls (HC), 38 patients with PD, 22 with progressive supranuclear palsy (PSP) and 20 with multiple system atrophy (MSA, 13 with the parkinsonian variant, 7 with the cerebellar variant) were recruited. The local institutional review board approved the study and written informed consent were obtained from all the participants. The SNc was manually segmented from neuromelanin-sensitive imaging for all subjects. Volume, corrected volumes, signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) values of the SNc were extracted from the SNc masks. A voxel-wise analysis was then performed based on the calculation of the neuromelanin SNR in the SNc and its subregions in template space. A mask corresponding to the SNc and a background mask which were previously calculated in template space based on neuromelanin-sensitive MRI of sixty-one HC, were used. 5 The SNc mask was manually segmented into three regions based on the functional subdivision of the SN in a posterolateral sensorimotor, anteromedial associative, and posteromedial limbic regions.6 These masks were applied to each subject’s T1-weighted image previously aligned to the brain template. Then, signal values in the whole SNc, in each SNc subregion and in the background masks were extracted and SNR values were calculated for each subject. Between-group differences in volume, corrected volume, SNR and CNR were evaluated by fitting general linear with age and sex for covariate adjustment, followed by post hoc pairwise comparisons between groups with correction for multiple testing.RESULTS
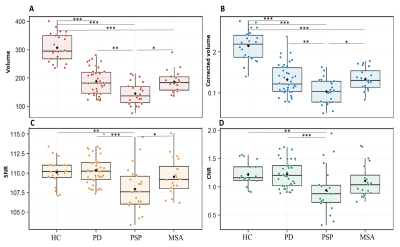
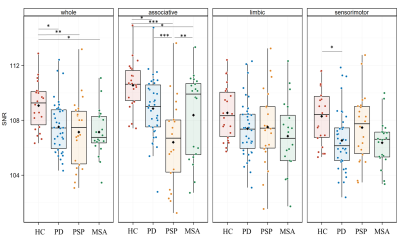
Results. Subject characteristics There were no between-group differences in sex proportion (p=0.40). Age was significantly different between groups (p=0.001), PSP patients being older than MSAc (p=0.02). UPDRS III scores (p=0.11) and disease duration (p=0.21) did not differ significantly between patient groups. Group differences in neuromelanin volume and signal based on manual segmentations There was a significant difference in SNc volume (p<0.001), corrected volume (p<0.001), SNR (F=7.50, df=3, p<0.001) and CNR (p<0.001) when comparing all groups. Post hoc comparisons showed that HC had higher SN volume and corrected volume than PD, PSP, MSA (p≤0.001) and higher SNR and CNR values than PSP (p<0.01). PSP patients had lower volume and corrected volume than PD (p<0.01 and p<0.001, respectively) and MSA (p<0.05), lower SNR values than PD (p<0.001) and MSA (p<0.05), and lower CNR values than PD (p<0.001) with a trend for MSA (p=0.05) (Figure 1). There were no differences between PD and MSA. Voxel-wise spatial distribution of neuromelanin changes Voxel-based analyses performed in the mask of the whole SNc of control subjects showed that SNR values were significantly different between groups (p=0.002). Post hoc comparisons indicated lower values in PD (p=0.016), PSP (p=0.002) and MSA (p=0.02) subjects versus HC without differences between patient groups (Figure 2). When comparing the three SN territories between groups, there was a significant effect of the group (p=0.001) and region (p<0.0001) factors with a significant interaction effect between Group and Region (p<0.0001). Post hoc comparisons showed that SNR values in the associative territory were lower in PSP (p<0.0001), PD (p=0.04) and MSA (p=0.03) subjects than in HC, and lower in PSP versus PD (p<0.0001) and MSA (p=0.01) subjects. In the sensorimotor territory, SNR values were lower in PD than in HC (p=0.03), and also lower in MSA patients than in HC but the difference did not reach significance (p=0.08). There were no significant differences in the limbic territory or between PD and MSA (Figure 2).DISCUSSION & CONCLUSION
Discussion and conclusion This study provides the first voxel-based MRI comparison of the topography of neuromelanin changes in parkinsonism. We confirm that neuromelanin volume and signal were reduced in parkinsonian disorders. The spatial pattern of changes differed between PSP and synucleinopathies. Compared to HC, PD and MSA, PSP had greater changes in the associative region. SNR in the sensorimotor territory was preserved in PSP, but reduced in PD patients and there was a trend in MSA patients. There was no significant difference between MSA and PD. These nigral topographical differences are consistent with the topography of the extra-nigral involvement in parkinsonian syndromes.Acknowledgements
No acknowledgement found.References
1. Dickson DW. Parkinson’s Disease and Parkinsonism: Neuropathology. Cold Spring Harb Perspect Med [online serial]. 2012;2.
2. Dickson DW. Neuropathology of Parkinson disease. Parkinsonism Relat Disord. 2018;46 Suppl 1:S30–S33.
3. Levin J, Kurz A, Arzberger T, Giese A, Höglinger GU. The Differential Diagnosis and Treatment of Atypical Parkinsonism. Dtsch Arzteblatt Int. 2016;113:61–69.
4. Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain J Neurol. 1999;122 ( Pt 8):1421–1436.
5. Biondetti E, Gaurav R, Yahia-Cherif L, et al. Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson’s disease. Brain J Neurol. Epub 2020 Aug 28. 6. Zhang Y, Larcher KM-H, Misic B, Dagher A. Anatomical and functional organization of the human substantia nigra and its connections. eLife. 6:e26653.
Figures