2376
Clinically uncertain parkinsonism shows nigral depigmentation in the presence and absence of dopaminergic deficit: a multimodal imaging study1Mental Health and Clinical Neuroscience Unit, University of Nottingham, Nottingham, United Kingdom, 2Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 3NIHR Nottingham Biomedical Research Centre, University of Nottingham, Nottingham, United Kingdom, 4Division of Neurology, Imperial College London, London, United Kingdom, 5Nuclear Medicine department, Royal Brompton & Harefield hospitals, London, United Kingdom, 6NHLI Department, Imperial College London, London, United Kingdom, 7Nottingham University Hospitals, Nottingham, United Kingdom, 8Department of Neurology and Neurosciences, Clínica Universidad de Navarra, Pamplona-Madrid, Spain, 9Department of Radiology, Cardiff and Vale University Health Board, Cardiff, United Kingdom
Synopsis
Brain dopamine transporter (DAT) imaging with 123I-FP-CIT SPECT has been used for detecting striatal deficits in clinically uncertain parkinsonism (CUP). Neuromelanin(NM)-MRI assesses nigral depigmentation with high accuracy in confirmed PD; but its diagnostic value in CUP is unclear. Here, we compare nigral NM in CUP with (DAT+) or without (DAT-) striatal dopaminergic deficits, to established PD and healthy controls. CUP with DAT+ had more nigral NM deficits (vs. controls and DAT-CUP) but were less pronounced than in established PD. Interestingly, DAT- cases showed ventrolateral nigral deficits. Our results suggest that nigral depigmentation possibly starts before striatal dopaminergic decline.
Introduction:
Parkinson’s disease (PD) is the second most common neurodegenerative disease. It is characterised by bradykinesia, tremor at rest, and rigidity. Its diagnosis in patients with some motor symptoms of PD lookalikes can be difficult especially at the early stage (clinically uncertain parkinsonism, CUP), and the dopamine transporter (DAT)-specific PET/SPECT scanning of the striatum may be needed for assistance. Yet, DAT imaging is available only in a few clinical settings in the world. To some extent, this is due to the high associated costs, and risks relevant to the radioactive aspect of the procedure. Additionally, DAT-specific imaging can be inconclusive in a few cases of questionable Parkinsonism (described in the literature as SWEDD: scans without evidence of dopaminergic deficits). To resolve those queries, clinicians usually opt for long clinical follow-ups, trial(s) with licensed dopaminergic drugs, or repeat(s) of DAT imaging. However, these strategies are ineffective and conversely, they increase the costs and risks of the diagnostic process even more.Imaging of the substantia nigra pars compacta (SNpc) with neuromelanin-sensitive MR (NM-MRI) offers high diagnostic accuracy to distinguish patients with established PD and healthy controls1-2. However, it is currently unknown whether NM-MRI can serve as an early diagnostic marker of CUP. To address this question, the aim of this study is to assess the nigral depigmentation level in subjects with CUP against established PD and age-matched healthy controls (HC) using NM-MRI.
Methods:
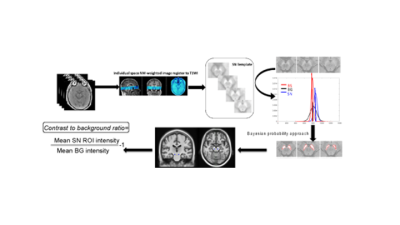
65 participants with CUP were recruited as part of a multi-centre longitudinal imaging study (N3iPD – https://clinicaltrials.gov/ct2/show/NCT03022357). All underwent a DAT-specific SPECT scan and a NM-MRI scan during the diagnostic period. Each DAT-specific scan was assessed visually by two experienced raters. Retrospective imaging data from two control groups were included for comparison. Those included 32 patients with clinically confirmed Parkinson’s disease (16 males; age mean±std 70.5±8.9 years) and 31 HCs (17 males; age 68.3±8.5 years). All participants underwent the same NM-MRI acquisition protocol: TR/TE= 38.4/3 milliseconds; flip angle, 20; slice thickness, 2mm; Field of view, 19.2; voxel size: 0.375´0.375´2mm; 30 slices. MR images were processed following a template-based in-house automated pipeline (Figure 1). Spherical ROIs (kernel=3 mm radius) were defined in the bilateral posterior and anterior SNpc, and their contrast-to-background ratios were calculated. Pairwise statistical analyses were conducted for group comparisons.Results:
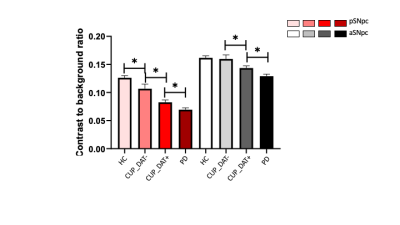
Brain SPECT imaging with 123I-FP-CIT: The majority of the CUP group showed reduced striatal DAT (CUP_DAT+) uptake (n=49/65 [75%], 31 males, age mean±std 67.2±12.0 years) while 16 did not (CUP_DAT-, 8 males, age: 72.4±8.6 years).NM-MRI: For both posterior and anterior SNpc, participants with CUP_DAT+ stood between the patients with established PD and the HC, showing higher contrast than those with established PD, and lower contrast than controls. Also, CUP_DAT+ had significant NM contrast losses in these SNpc subregions, compared with CUP_DAT-. NM contrast for individuals with CUP_DAT- did not differ from HC in the anterior SNpc but was reduced in the posterior SNpc. The PD group showed the lowest contrast ratio in both parts of SNpc (Figure 2).
Discussions:
Abnormal DAT-specific imaging (DAT+) in the striatum suggests severe degeneration related to parkinsonism. In the present study, we found lower NM contrast in the CUP_DAT+ versus HC, suggesting that NM-MRI can capture nigral NM losses in a group of patients with suspected PD. This is consistent with NM-MRI evidence in people at risk for PD i.e. people with rapid eye movement sleep behavior disorder3,4. NM contrast was higher in the CUP_ DAT+ group as compared to patients with confirmed PD, supporting previous findings in NM metrics that associate nigral depigmentation with PD severity and ageing markers 1,5-6.A DAT-specific scan of the normal spectrum (DAT-) supports high striatal dopaminergic integrity and distinguishes cases with essential tremor, drug-induced, and arguably vascular parkinsonism from patients with idiopathic PD, dementia with Lewy body, and related disorders. In this regard, we expected ‘normal’ NM contrast in CUP_DAT-. By surprise, our dataset revealed low NM contrast in the posterior SNpc at variance to what we observed in age-matched healthy controls. This may be explained by the heterogeneity in our CUP group: we had participants with normal DAT-specific uptake as well as people with very early and relatively little loss of nigral cells (‘true’ SWEDD) 7-8, implying that quantitative NM-MRI may be more sensitive in measuring presynaptic nigrostriatal deficits than only visual evaluation of DAT-specific imaging. In addition, this difference was only found in the posterior but not anterior SNpc, which concords with the expected spatio-temporal progression of depigmentation along the posterior->anterior SNpc axis, similar to previous evidence 6,9. However, longitudinal and independent observations of these imaging findings, including quantitative measures of the striatal DAT-specific SPECT scan, and clinical development and characteristics, as a part of the ongoing data collection, are still needed for further validation.
Conclusion:
Our NM-MRI findings in clinically uncertain parkinsonism demonstrate an intermediary NM phenotype between health and established disease, and notably early depigmentation in CUP without dopaminergic deficits. We recommend NM-MRI as a powerful diagnostic tool of PD and its utility for studying prodromal PD in the future.Acknowledgements
The Michael J. Fox Foundation for funding this project. Weston Brain Institute for funding Y. X..References
1. Schwarz ST., Xing Y. Tomar P, Bajaj N, Auer, DP. In vivo assessment of brainstem depigmentation in Parkinson disease: Potential as a severity marker for multicenter studies Radiology, 2017.
2. Schwarz ST, Rittman T, Gontu V, Morgan PS, Bajaj N, Auer DP. T1-weighted MRI shows stage-dependent substantia nigra signal loss in Parkinson's disease. Mov Disord. 2011 Aug 1;26(9):1633-8. doi: 10.1002/mds.23722. Epub 2011 Apr 12. Erratum in: Mov Disord. 2012 Feb;27(2):335. PMID: 21491489.
3. Pyatigorskaya N, Gaurav R, Arnaldi D, Leu-Semenescu S, Yahia-Cherif L, Valabregue R, Vidailhet M, Arnulf I, Lehéricy S. Magnetic Resonance Imaging Biomarkers to Assess Substantia Nigra Damage in Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Sleep. 2017 Nov 1;40(11). doi: 10.1093/sleep/zsx149. PMID: 28958075.
4. Emma Biondetti, Rahul Gaurav, Lydia Yahia-Cherif, Graziella Mangone, Nadya Pyatigorskaya, Romain Valabrègue, Claire Ewenczyk, Matthew Hutchison, Chantal François, Jean-Christophe Corvol, Marie Vidailhet, Stéphane Lehéricy, Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson’s disease, Brain, Volume 143, Issue 9, September 2020, Pages 2757–2770.
5. Quantifying the Severity of Parkinson Disease by Use of Dopaminergic NeuroimagingHiroto Takahashi, Yoshiyuki Watanabe, Hisashi Tanaka, Hideki Mochizuki, Hiroki Kato, Jun Hatazawa, and Noriyuki Tomiyama; American Journal of Roentgenology 2019 213:1, 163-168.
6. Gaurav, R., Yahia-Cherif, L., Pyatigorskaya, N., Mangone, G., Biondetti, E., Valabrègue, R., Ewenczyk, C., Hutchison, R.M., Cedarbaum, J.M., Corvol, J.-C., Vidailhet, M. and Lehéricy, S. (2021), Longitudinal Changes in Neuromelanin MRI Signal in Parkinson's Disease: A Progression Marker. Mov Disord, 36: 1592-1602.
7. Josephs KA, Matsumoto JY, Ahlskog JE. Benign tremulous parkinsonism. ArchNeurol 2006;63:354–7.8. Selikhova M, Kempster PA, Revesz T, et al. Neuropathological findings in benigntremulous parkinsonism. Mov Disord 2013;2
8:145–52.9.Xing Y, Sapuan A, Dineen RA, Auer DP. Life span pigmentation changes of the substantia nigra detected by neuromelanin-sensitive MRI. Mov Disord. 2018 Nov;33(11):1792-1799. doi: 10.1002/mds.27502. Epub 2018 Nov 13. PMID: 30423212; PMCID: PMC6659388.
Figures