2374
Nigral Neuromelanin MRI Changes in Isolated Rapid Eye Movement Sleep Behavior Disorder Using Deep Learning1Movement Investigations and Therapeutics Team (MOV’IT), Paris Brain Institute (ICM), Paris, France, 2CENIR, Paris Brain Institute (ICM), Paris, France, 3Department of Neuroradiology, Pitié-Salpêtrière Hospital, AP-HP, Paris, France, 4INSERM, Clinical Investigation Center for Neurosciences (CIC), Paris Brain Institute (ICM), Paris, France, 5Sleep Disorders Unit, Pitié-Salpêtrière Hospital, AP-HP, Paris, France, 6Department of Neurology, Pitié-Salpêtrière Hospital, AP-HP, Paris, France
Synopsis
Isolated REM sleep behavior disorder (iRBD) is considered a prodromal stage of parkinsonism. Neurodegenerative changes in the substantia nigra pars compacta (SNc) in parkinsonism can be detected using neuromelanin-sensitive MRI. In this cross-sectional, observational, case-control study, we investigated changes in neuromelanin in participants with iRBD compared to Parkinson’s disease and healthy volunteers in the SNc segmented automatically using convolutional neural network-based U-net architecture. The iRBD participants had reduced neuromelanin content at an intermediate level between the values in HV and PD patients.
INTRODUCTION
Rapid eye movement (REM) sleep behavior disorder (RBD) comprises REM sleep parasomnia, featuring unpleasant dreams and/or acting out the dreams in real. There is also a loss of normal muscle atonia during REM sleep.1 RBD is common in patients with synucleinopathies and uncommon in patients with other neurodegenerative disorders.1 Typically, RBD can be categorized as isolated (iRBD) or secondary (when a cause is already known). Participants with iRBD manifest RBD alone without concomitant parkinsonism.1 Parkinson’s disease (PD) is a long-term gradually progressing neurodegenerative disorder characterized by the gradual loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) resulting in striatal dopamine depletion.2,3 Studies have demonstrated that most iRBD subjects will acquire PD or dementia with Lewy bodies with a median conversion time of 7 years.4,5 At PD onset, 30% to 60% of the dopaminergic neurons in the substantia nigra pars compacta (SNc) are already lost.2,3 Given that iRBD is considered a prodromal stage of parkinsonism, a mild SNc damage was evidenced in a small group of patients4,5 using neuromelanin-sensitive MRI.6,7 In this study, we designed a convolutional neural network-based8,9,10 U-net architecture11 to automatically segment the SNc with an aim to investigate the nigral neuromelanin changes in a larger group of participants with iRBD as compared to PD patients and healthy volunteers (HV).MATERIALS AND METHODS
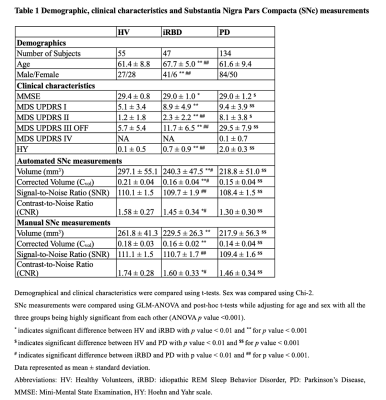
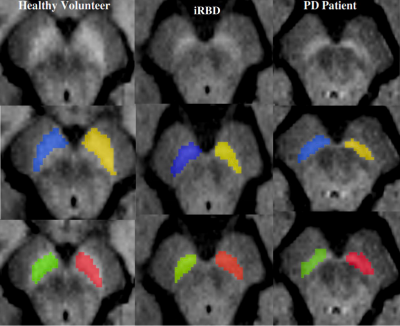
Participants were scanned at 3 Tesla MRI using a 64-channel head coil. The MRI protocol included 3D T1-weighted and neuromelanin images as part of the ICEBERG study (ClinicalTrials.gov: NCT02305147). The local ethics committee approved this study and all subjects provided written informed consent (IRB of Paris VI, RCB 2014-A00725-42). Using FreeSurfer viewer (v5.3.0), the SNc manual segmentations were performed by two experts who were blind to the clinical status of the subject. Thereafter automatic SNc segmentations were performed using our proposed deep learning model NigraNet that is a modified U-net architecture. The deep learning pipeline was implemented in Python 3.6.0 using Scikit-learn (v0.21.0) and Keras library (v2.2.4) with the TensorFlow framework (v1.14) at the backend. The model was trained on NVIDIA-quadro P6000 (v418.67) with CUDA 10.1 and 24GB GPU memory. NigraNet was trained on 54, validated on 6, and tested on the remaining 176 images. The SNc measurements comprised volumes (Vol); corrected volume (Cvol = Vol/total intracranial volume) to normalize for the head size; signal-to-noise ratio; and contrast-to-noise ratio.Furthermore, a one-way general linear model analysis of variance adjusted for age and sex and post-hoc t-tests were performed. Receiver operating characteristic (ROC) analysis was calculated to assess the diagnostic performance. Dice similarity coefficient was used to compare the NigraNet and manual segmentation performance.
RESULTS AND DISCUSSION
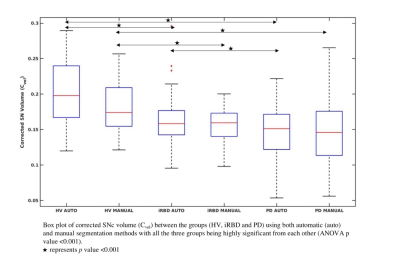
We analyzed 236 participants including 47 iRBD (age: 67.7 ± 5.0 years, MDS UPDRS OFF score: 11.7 ± 6.5), 134 PD (age: 61.6 ± 9.4 years, MDS UPDRS OFF score: 29.6 ± 7.9, disease duration: 1.5 ± 1.0 years), and 55 HV (age: 61.4 ± 8.8 years, MDS UPDRS OFF score: 5.6 ± 5.3). Participants with iRBD were older than HV (p<0.001) and there was no significant difference in age between PD and HV. There was a larger proportion of males among iRBD and PD patients than in HV (χ2=16.484, p<0.001). All the SNc measurements significantly differed between the three groups. Using both automatic and manual methods, we observed a significant sex effect in Cvol between the three groups, and in CNR between iRBD and PD groups. Furthermore, all the SNc measurements were significantly lower in PD than in both iRBD and HV, and in iRBD than in HV except for SNR that did not differ between iRBD and HV. Using NigraNet, for iRBD and HV, areas under the curve of the ROC analyses were Vol = 0.78, Cvol = 0.79, SNR = 0.56 and CNR = 0.63, and for PD and HV, Vol = 0.83, Cvol = 0.85, SNR = 0.79 and CNR = 0.77. Between-group differences and the ROC analysis results were similar using the manual method.Dice similarity coefficient of 0.80 was obtained between NigraNet and manual SNc segmentations demonstrating a high reproducibility between both the methods.NigraNet demonstrated significant differences between both iRBD and PD, and between iRBD and HV separately for all the SNc measurements. Volumes and signal changes were at an intermediate level between values in HV and PD patients.
The SNc changes observed using NigraNet were comparable to the established ground truth that was the manual method. Furthermore, our model needed a small training dataset, and the untrained and non-validated testing dataset of 176 subjects was more than three times higher than the training dataset suggesting the robustness of our model that has the potential to segment untrained external neuromelanin cohorts from other scanners as well in the future.
CONCLUSION
In this study, we demonstrated that using a robust fully automated SNc segmentation model NigraNet, iRBD presents reduced nigral neuromelanin content in a large number of subjects. Henceforth, neuromelanin-sensitive MRI, coupled with our fast and user-independent NigraNet, has the potential to be the future biomarker for clinical trials of disease-modifying treatments and could enable reproducible and fast SNc segmentation in large patient cohorts.Acknowledgements
The authors would like to thank Energipole (M. Mallart), M.Villain and Société Française de Médecine Esthétique (M. Legrand) for unrestricted support for Research on Parkinson’s disease.
We would also like to thank all of the participants involved in the ICEBERG study (ClinicalTrials.gov:NCT02305147), who have helped to make this research possible.
References
- Iranzo A, Fernández-Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: Study in 174 patients. PLoS One 2014;9(2)
- Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat. Rev. Dis. Prim.2017;3:1–21.
- Kish SJ, Shannak K, Hornykiewicz O. Uneven Pattern of Dopamine Loss in the Striatum of Patients with Idiopathic Parkinson’s Disease. N. Engl. J. Med. 1988;318(14):876–880.
- Iranzo A, Lomeña F, Stockner H, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: A prospective study [Internet]. Lancet Neurol. 2010;9(11):1070–1077.Available from: http://dx.doi.org/10.1016/S1474-4422(10)70216-7
- Pyatigorskaya N, Gaurav R, Arnaldi D, et al. Magnetic Resonance Imaging Biomarkers to Assess Substantia Nigra Damage in Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Sleep 2017;40(11):1–8
- Sulzer D, Cassidy C, Horga G, et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease [Internet]. Npj Park. Dis. 2018;4(1):11.Available from: http://www.nature.com/articles/s41531-018-0047-3
- Sasaki M, Shibata E, Tohyama K, et al. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease. Neuroreport 2006;17(11):1215–1218
- Le Berre A, Kamagata K, Otsuka Y, et al. Convolutional neural network-based segmentation can help in assessing the substantia nigra in neuromelanin MRI. Neuroradiology 2019;61(12):1387–1395.
- Krizhevsky A, Sutskever I, Hinton GE. ImageNet Classification with Deep Convolutional Neural Networks. Commun. ACM 2012;60(6):84–90.
- Le Cun , B. Boser , J. S. Denker , D. Henderson , R. E. Howard , W. Hubbard LDJ. LeNet-5: Handwritten Digit Recognition with a Back-Propagation Network [Internet]. Adv. Neural Inf. Process. Syst. 1998;39(1pt2):149.Available from: http://dl.acm.org/citation.cfm?id=109230.109279
- Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. Lect Notes Comput Sci (including Subser Lect Notes Artif Intell Lect Notes Bioinformatics). 2015;9351:234-241. doi:10.1007/978-3-319-24574-4_28
Figures