2372
Parkinson’s disease local atrophies measured in the striatum in vivo explain patients’ asymmetric motor symptoms and dopamine loss
Elior Drori1, Shai Berman1, and Aviv Mezer1
1The Edmond and Lily Safra Center for Brain Sciences, The Hebrew University of Jerusalem, Jerusalem, Israel
1The Edmond and Lily Safra Center for Brain Sciences, The Hebrew University of Jerusalem, Jerusalem, Israel
Synopsis
The striatum is a heterogeneous brain structure with microstructural gradients along its main axes. Changes in its organization are associated with Parkinson’s disease (PD). Yet the spatial variability in the human striatum is not well characterized and is mostly limited to animal and postmortem studies. We developed a non-invasive MRI method for measurement of microstructural gradients along axes of the striatum in individuals in vivo. Using clinical data, we found a T1w/T2 gradient along the anterior-posterior axis of the putamen, which is decreased in PD. This decrease explains the patients’ asymmetries in dopamine loss and motor symptoms.
Introduction
The striatum is involved in motor control and goal-directed behavior, which are affected in neurodegenerative diseases such as Parkinson’s disease1. While the typical striatal tissue is characterized by heterogeneous organization, spatially-dependent changes are apparent in PD, mainly along the striatum’s anterior-posterior axis, with acute degeneration in posterior parts of the putamen2,3. This degeneration is characterized by the depletion of dopaminergic innervation to the striatum, often asymmetrically between hemispheres. However, the striatal tissue composition is studied mainly using invasive animal research and human postmortem methods, and the relationship between dopamine loss, tissue deterioration and motor disfunction in PD is not well characterized in vivo. We have recently developed a non-invasive tool for quantifying structural heterogeneity along axes of the human striatum of individuals, using quantitative MRI4. Here, we use this method to detect abnormal, spatially dependent PD-related changes in the striatum, using a semi-quantitative MRI contrast, generated from widely available clinical data. We further investigate the relationship between local alterations and dopamine loss, measured by SPECT DaTSCAN, as well as the motor symptoms assessed through the MDS-UPDRS III5. We show that asymmetries in structural deterioration are associated with dopamine loss in the putamen ipsilaterally, and with motor disfunction contralaterally. Hence, we provide a non-invasive method to link structure, function, and behavior in individual PD patients. Since our approach exploits MRI data that is widely used in clinical settings, it may prove useful for wide clinical and diagnostic applications.Methods
Subjects and data. Data obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). We analyzed 99 older, early-stage PD patients (aged 65 ± 6 years, range 55-76; 32 female), Hoehn and Yahr Scale’s stage 1 (N=40) or stage 2 (N=59). 46 healthy controls were matched for age and sex (aged 65 ± 6 years, range 55-76; 17 female). T1w and T2w images were acquired for all subjects with a 3T MRI scanner. Using these images, we generated a semi-quantitative T1w/T2w contrast that minimized the shared bias of the weighted images. Subcortical brain segmentation was done using FSL FIRST6.Dopamine transporter binding ratio. SPECT DaTSCAN was performed on all subjects for assessment of dopamine transporter (DAT) deficit, and the striatal binding ratio (SBR) was calculated for the putamen and caudate. Dopamine loss asymmetry was calculated for each subject as the left-minus-right SBR.
Motor assessment. Motor symptoms were assessed through MDS-UPDRS-III. The motor symptoms asymmetry was calculated as the raw score for left body-side items minus the raw score for right body-side items.
Structural gradients of the striatum. We developed4 an automated MATLAB-implemented tool to compute the three main orthogonal axes of each individual’s subcortical structures, using SVD. The structure (e.g., left putamen) is then segmented to equally spaced segments along each axis, and the MRI values are then sampled along them. This yield structural MRI profiles which are functions of position along the main axes of the subject’s striatum.
Results
In healthy controls (HC; N=46) we found a robust, increasing T1w/T2w gradient along the anterior-posterior (AP) axis of the left and right putamen (mixed-effects linear model, p <10-67; Fig. 1a). This gradient was correlated with quantitative R1 AP gradient that we have found previously4, suggesting that T1w/T2w gradients in the striatum reflect spatial variation in tissue biophysical properties (Fig. 1b). In early-stage PD patients (N=99), however, we found alterations in the structural gradient, expressed as a reduction in the posterior subregions of the putamen (p <.05; Fig. 2a). On the single-subject level, we found that interhemispheric asymmetry in T1w/T2w intensity was positively correlated with ipsilateral asymmetry in dopamine transporter striatal binding ratio (DaT SBR). Namely, a greater T1w/T2w decrease in the posterior putamen in one hemisphere relative to the other was associated with a greater decrease of the DaT SBR in the putamen of that same hemisphere (R2 =.25, p <10-7; Fig. 2b). Moreover, we found that the gradient asymmetry was positively correlated with contralateral asymmetry in the patients’ motor symptoms, as assessed using the MDS-UPDRS III (R2 =.25, p <10-7; Fig. 2c, e). Interestingly, this relationship could not be found using conventional whole-ROI mean statistics (R2 =.01, p =0.4; Fig. 2d). Hence, using our spatial approach with non-invasive MRI clinical data, we found a novel in vivo correlate for PD, associated with the individual patients’ underlying dopaminergic loss and the motor symptomatology.Conclusions
Our study suggests that tissue microstructure gradients can be detected along the main axes of the striatum using T1w/T2w contrast. Importantly, these gradients show a local alteration in the posterior putamen in early-stage PD patients. This PD-related structural abnormality is associated with both the asymmetric decrease in dopamine transporter binding ratio, measured with SPECT, and the PD-related asymmetric motor decline, assessed with MDS-UPDRS III. Hence, our approach opens a venue towards an early non-invasive characterization of PD neural correlates in individual patients and may promote early diagnosis and personalized medicine in PD. Future research using quantitative MRI measurements may shed further light on the biological mechanisms underlying the structural changes in PD, as well as in other basal-ganglia disorders.Acknowledgements
No acknowledgement found.References
- Redgrave, P. et al. Goal-directed and habitual control in the basal ganglia: Implications for Parkinson’s disease. Nature Reviews Neuroscience vol. 11 760–772 (2010).
- Moratalla, R. et al. Differential vulnerability of primate caudate-putamen and striosome-matrix dopamine systems to the neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine. Proc. Natl. Acad. Sci. U. S. A. 89, 3859–3863 (1992).
- Garnett, E. S., Lang, A. E., Chirakal, R., Firnau, G. & Nahmias, C. A rostrocaudal gradient for aromatic acid decarboxylase in the human striatum. Can. J. Neurol. Sci. 14, 444–447 (1987).
- Drori, E., Filo, S. & Mezer, A. Measuring Biological Gradients along the Human Dorsal Striatum in vivo using Quantitative MRI. in ISMRM Annual Meeting (2020).
- Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
- Patenaude, B., Smith, S. M., Kennedy, D. N. & Jenkinson, M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56, 907–922 (2011).
Figures
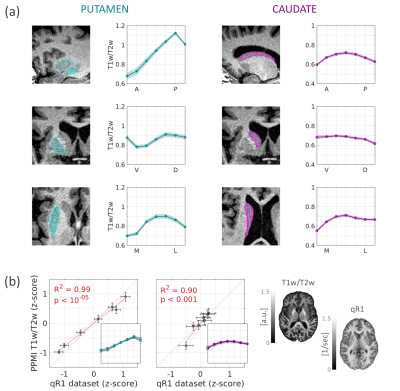
Fig.1 – T1w/T2w gradients in healthy old adults.
(a) T1w/T2w profiles along the main axes of the left putamen
and caudate in healthy adults (Mean ±1 SEM). Insets: axes segmentations on a
T1w image. (b) T1w/T2w profiles in the AP axes of the putamen and
caudate are correlated with the qR1 profiles measured in a separate dataset (age
matched, N=17). Insets: z-scored T1w/T2w (colored) and qR1 (grey) profiles. Shown
on the right are representative axial slices of T1w/T2w and qR1 in healthy
subjects.
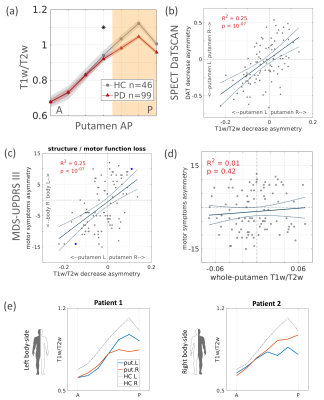
Fig.2 – Putamen gradients reveal a decrease in PD, correlated
with dopamine and motor deficits.
(a) Putamen AP gradient in PD is decreased posteriorly
compared to HC. *p <.05 (b-c) Posterior gradient reduction is
correlated with (b) ipsilateral DAT asymmetry, and (c) contralateral symptom
asymmetry. Blue highlight: 2 patients shown in panel e. (d) Whole-ROI measurement
does not explain symptom asymmetry. (e) Two PD patients with symptoms
affecting the left or right body-sides, demonstrate asymmetric gradient
decrease and a contralateral association with the symptom laterality.
DOI: https://doi.org/10.58530/2022/2372