2354
DeepFLAIR: a neural network approach to mitigate signal loss in temporal lobe regions of 7 Tesla FLAIR images1School for Mental Health and Neuroscience, Maastricht University, Maastricht, Netherlands, 2Department of Radiology & Nuclear Medicine, Maastricht University Medical Centre, Maastricht, Netherlands, 3Department of Cognitive Neuroscience, Maastricht University, Maastricht, Netherlands, 4Scannexus, Maastricht, Netherlands, 5Academic Centre for Epileptology, Kempenhaeghe/Maastricht University Medical Centre, Heeze/Maastricht, Netherlands, 6Department of Neurosurgery, Maastricht University Medical Centre, Maastricht, Netherlands
Synopsis
In this study we aimed to improve the 7T FLAIR image quality, especially within the temporal lobe regions which are often attenuated due to field inhomogeneities. A neural network using MP2RAGE and T2-weighted images as inputs was set up to generate a new FLAIR-like image. The training was performed on the extratemporal-lobe voxels of the acquired 7T FLAIR image. The deepFLAIR showed a significant improvement in the signal-to-noise ratio and contrast-to-noise ratio in the temporal lobe regions in a number of cases. This study showed the potential to generate FLAIR-like images with reduced inhomogeneity artifacts and improved image quality.
Introduction
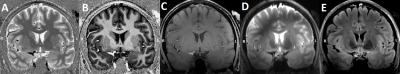
Epilepsy affects approximately 1% of the world-wide population, with temporal lobe epilepsy (TLE) being the most common focal epilepsy type1. In recent years, Ultra High Field Magnetic Resonance Imaging (UHF MRI) has been shown to improve the detection of subtle potential epileptogenic lesions in patients with focal epilepsy2,3. Unfortunately, the higher magnetic field strength in UHF imaging introduces increased inhomogeneities in B0 and B1 fields leading to image artifacts and signal attenuations3,4. The T2-weighted FLAIR sequence is of particular interest to clinicians involved in the presurgical workup for epilepsy surgery due to its strong contrast and suppressing the cerebral spinal fluid, however it uses an inversion pulse and refocusing pulses which suffer from these inhomogeneities4,5. For the FLAIR sequence, the (inferior) temporal lobe area is often affected by signal attenuation (see Figure 3A, 2C). In this study, we attempt to improve the signal loss due to UHF inhomogeneities in temporal regions by creating a neural-network-generated FLAIR image (“deepFLAIR”) which could potentially reveal subtle temporal neocortical lesions.Methods
Eight patients (mean age 31y; range 21-46y; 5 females) with chronic, drug-resistant epilepsy with a suspected epileptogenic zone (2 patients with 3T-positive lesions) were selected for this study. All patients were scanned on a 7T MRI (Siemens Healthcare, Erlangen, Germany) with a 32-element channel phased-array head coil and bilaterally positioned dielectric pads. The following structural brain scans were acquired. MP2RAGE (0.7x0.7x0.7mm; TE=2.47ms; TR=5030ms; TI1=900ms; TI2=2750ms), T2-weighted (transverse slices, 0.6x0.6x2.0mm; TE=283ms; TR=4000ms), SPACEFLAIR (0.8x0.8x0.8mm; TE=303ms; TR=8000ms; TI=2330ms). All scans were coregistered to the quantitative MP2RAGE T1 map and the T1 map was segmented into grey matter (GM) and white matter (WM).A non-linear regression multilayer perceptron (MLP) has been set up to reconstruct the 7T FLAIR images and boost the signal in the temporal lobe areas which are being affected by the signal attenuations due to the field inhomogeneities. The MLP was trained on 4 input images (see Figure 1A-D) and consisted of 4 hidden layers (16; 64; 32; 8 neurons). The output layer consisted of a single neuron. The MLP works on a voxel-wise basis, meaning that each voxel in the generated FLAIR-like image is determined by the corresponding voxels from the input images. The training was done on slices outside the temporal lobe region. The training lasted for 10 epochs with batch size=100. After the training, the entire scanned area was reconstructed using the trained model to form the deepFLAIR image.
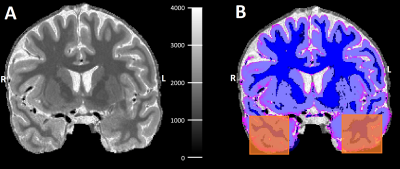
Right and left temporal lobe regions-of-interest (ROIs) sized 28x28x28mm were manually delineated to facilitate the evaluation (see Figure 2). The quality of the output image was evaluated by calculating the signal-to-noise ratio (SNR) of the WM and contrast-to-noise ratio (CNR) between the GM and WM in the temporal lobe ROIs using the following equations:
$$SNR = \frac{\mu}{\sigma} \text{ }\text{ }\text{ }\text{ }\text{ }\text{ }\text{ }\text{ }\text{ }\text{ }\text{ }\text{ }\text{ }\text{ }\text{ }\text{ } CNR = \frac{\left| \mu_{gm} - \mu_{wm} \right|}{\sqrt[]{\frac{\sigma_{gm}^{2} + \sigma_{wm}^{2}}{2}}}$$
where µ and σ are the mean voxel intensity and the standard deviation from the selected tissue (GM or WM) within the ROI, respectively. The SNR and CNR from right and left ROIs were statistically compared via a Wilcoxon signed-rank test between the originally acquired 7T FLAIR and the deepFLAIR images.
Results
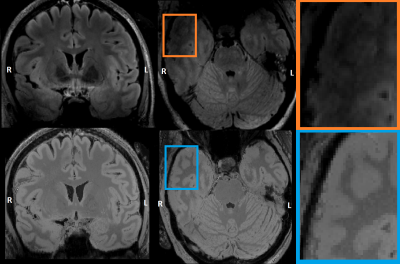
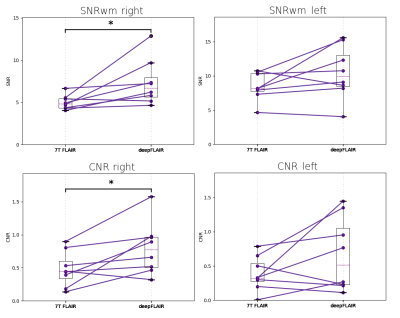
Figure 3 shows the generated deepFLAIR image compared with the acquired 7T FLAIR images for a representative subject. When visually assessing the acquired images, the right temporal lobes seemed to be more affected by the magnetic inhomogeneities than their left counterparts. The statistical test revealed a significant improvement in the deepFLAIR image in SNRWM (p=0.016) and CNR (p=0.023) in the right temporal lobe area. The deepFLAIR approach showed an increase in both SNRWM and CNR for 10 out of 16 temporal lobes. The SNRWM and CNR results were visualized in a paired line plot in Figure 4.Discussion & Conclusion
This study demonstrated a concept of neural-network-informed improvement of the 7T FLAIR image contrast. A statistically significant improvement was found for both SNRWM and CNR in the right temporal lobes. This suggests that the deepFLAIR approach could have a significant impact in the more severely attenuated temporal lobe regions and therefore facilitate a more confident diagnosis. The per-subject nature of the deepFLAIR provides independence from an extensive subject database but also limits the training data to merely a single brain. The concept seems promising and utilizing more sophisticated network architectures, alterations of the training data or extension across multiple subjects could result in further improvements. This work also needs to be expanded with more varied cases including specific types of epileptogenic lesions and careful prior evaluation by an independent neuroradiologist. The deepFLAIR, despite its qualities, should still be interpreted with care. The main limitation is the lack of a golden standard in the temporal lobe regions, which could be potentially provided with the use of Universal Pulses4.This conceptual study presented a possibility to generate FLAIR-like images with reduced inhomogeneity artifacts in the inferior anterior/lateral temporal lobe and overall improved tissue contrast. Further optimization of the network and the training dataset might provide a significant boost in the image quality and its ability to fully reconstruct the attenuated temporal lobe areas.
Acknowledgements
Funded by the Dutch Epilepsy Foundation, grant nr. 20-09References
1. Fiest KM, Sauro KM, Wiebe S, et al. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies [published correction appears in Neurology. 2017 Aug 8;89(6):642]. Neurology. 2017;88(3):296-303. doi:10.1212/WNL.0000000000003509
2. Wang I, Oh S, Blümcke I, et. al. Value of 7T MRI and post-processing in patients with nonlesional 3T MRI undergoing epilepsy presurgical evaluation. Epilepsia. 2020 Nov;61(11):2509-2520. doi: 10.1111/epi.16682.
3. Opheim G, van der Kolk A, Markenroth Bloch K, et. al. 7T Epilepsy Task Force Consensus Recommendations on the Use of 7T MRI in Clinical Practice. Neurology. 2021 Feb 16;96(7):327-341. doi: 10.1212/WNL.0000000000011413
4. Gras V, Mauconduit F, Vignaud A, et. al. N. Design of universal parallel-transmit refocusing kT-point pulses and application to 3D T2-weighted imaging at 7T. Magn. Reson. Med. 2018; 80: 53-65. https://doi.org/10.1002/mrm.27001
5. van Lanen RHGJ, Colon AJ, Wiggins CJ, et. al. Ultra-high field magnetic resonance imaging in human epilepsy: A systematic review. NeuroImage: Clinical. 2021;30. 102602. https://doi.org/10.1016/j.nicl.2021.102602
Figures