2338
DTI and fMRI Biomarkers in a Pig Model of Pediatric Traumatic Brain Injury1Biomedical Engineering, Michigan State University, East Lansing, MI, United States, 2Radiology, Baylor College of Medicine, Houston, TX, United States, 3Neuroscience, Michigan State University, East Lansing, MI, United States, 4Radiology, Michigan State University, East Lansing, MI, United States, 5Veterinary Medicine, Michigan State University, East Lansing, MI, United States
Synopsis
The goal of this work is to identify MRI biomarkers of mild traumatic brain injury (mTBI) using a pig model of pediatric concussion. We have found changes in the brain using DTI and fMRI in the days and weeks immediately following injury. We also collected data from a battery of cognitive, emotional, and motor assessments. Together, we anticipate these data will help us to identify biomarkers of injury that will improve diagnostics of children to decrease the risk of post-injury complications.
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disabilities in children in the United States. Evidence suggests that even after so called “clinical recovery”, over 40% of children with mild TBI (concussion) endure emotional, cognitive, and neurological impairments that persist for months and even decades after the injury. MRI is the gold standard to visualize structural and functional changes associated with TBI, but often no abnormalities are observed after mTBI in children. This along with partial and subjective reports of symptoms, results in children not receiving the appropriate guidelines and therapies to minimize future complications. A promising body of work performed mainly in adults suggest that advanced MRI modalities such as diffusion tensor imaging (DTI) and fMRI demonstrate patterns that correlate with symptom severity and recovery in mTBI (1-5). Despite its potential utility, only a few DTI studies have investigated these diffusion metrics following mTBI in youth (6, 7) Our work is designed to identify MRI biomarkers that will assist in the diagnosis of mTBI in children. We are employing unprecedented multimodal measurements in a pig model that demonstrates the phenotype associated with the injury. Pigs resemble humans in many aspects that are critical to injury biomechanics and pathophysiology, and they are an established experimental model for head injury (8-15). Recently, we characterized the executive functions, learning and memory, circadian rhythms, gait analysis, and level of motor activity in healthy Yucatan minipigs throughout their development (16). In this study, we have established a model of closed-head injury in adolescent Yucatan minipigs, and we characterized in vivo changes using DTI and fMRI in tandem with administering a battery of behavioral tests and implementing wearable biosensors to monitor preinjury and postinjury behavior.Methods
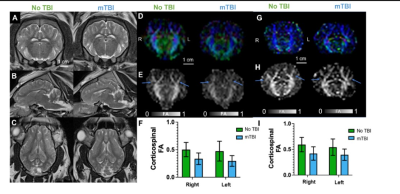
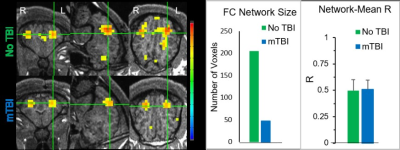
All experiments were approved by the institutional animal care and use committee. Closed-head injury to deliver mild TBI with a Head Injury Criterion (HIC) of 500 (17) was performed on 16-weeks old Yucatan minipigs under general anesthesia. A battery of behavioral tests was performed daily until pigs reached adulthood (36 weeks). MRI was performed at 48 h and 90 days after injury. Pigs were anesthetized and intubated during scanning. Anatomical imaging, DTI, and fMRI data were acquired on a Siemens MAGNETOM Espree 1.5T scanner. A 6-channel head/neck coil was be used. Pigs were placed in a prone position. DTI: TE/TR: 127/7900 ms; FOV:230 mm, voxel size (mm3): 2.0x2.0x2.0, 40 slices, 64 directions with b=1000 s/mm2. GE-EPI (rs-fMRI): TE/TR: 37/2000 ms. FOV:172 mm, voxel size (mm3): 2.7x2.7x2.7, 38 slices, 120 volume images. DTI data was processed using the Tortoise DTI processing pipeline for correcting distortion due to motion and eddy current diffusion artifacts (18). Automated methods for skull-stripping, rigid transformation, and brain angular adjustment was performed manually for this analysis using Tortoise and MATLAB algorithms developed in house. ROI of major tracks were drawn through contiguous slices using MRIStudio software (19) and fractional anisotropy (FA) maps were calculated. rs-fMRI data analysis: A seed was selected in the sensorimotor area to determine a functional connectivity (FC) network. The Pearson correlation coefficient (R) of a voxel’s signal time course with the seed’s signal time course was computed for all voxels, and thresholded with R>0.4 (P<6.0×10-6, N=120) to yield the FC networkResults
Our preliminary results show that mTBI induces a combination of behavior phenomics that includes personality changes, impaired learning and memory, impaired attention, changes in sleep patterns, increased anxiety and depression, decreased motor activity, and impaired gait. Multi-parametric MRI was performed 48 h and 90 days after injury. Macroscopic changes in brain tissue due to injury were not visible with conventional anatomical T1 and T2 weighted imaging. However, ROI analysis of DTI data processed via the Tortoise pipeline (18) yielded FA maps that demonstrated mean FA differences in prominent white matter tracts between the injured and uninjured pigs used in this study as shown in Figure 1. Resting-state fMRI analysis demonstrates that we also found reduced FC network size in sensorimotor network in mTBI pigs as shown in Figure 2.Discussion
Our model of closed-head pediatric mTBI in minipigs is unique and exceptional in many levels and will allow us to collect clinically relevant data and test new treatments. Similar to what is observed in the clinic, we did not detect any macrostructural changes with T1 and T2 MRI contrast, but we did detect decreased FA in the superior corticospinal tract, a prominent white matter tract directly beneath the site of injury in the injured animal. This tract has been demonstrated as a possible site of injury in children (4). We will apply rigorous statistical approaches to identify the MRI biomarkers that correlate with behavior.Acknowledgements
No acknowledgement found.References
1. A. Alivar et al., Relationship between DTI Brain Connectivity and Functional Performance in Individuals with Traumatic Brain Injury(.). Annu Int Conf IEEE Eng Med Biol Soc 2020, 3256-3259 (2020).
2. K. L. Main et al., DTI measures identify mild and moderate TBI cases among patients with complex health problems: A receiver operating characteristic analysis of U.S. veterans. Neuroimage Clin 16, 1-16 (2017).
3. S. Turner, R. C. Lazarus, D. W. Marion, K. L. Main, Molecular and DTI Biomarkers of Traumatic Brain Injury: Principles for Investigation and Integration. J Neurotrauma 10.1089/neu.2020.7259 (2021).
4. E. A. Wilde et al., Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Dev Neuropsychol 35, 333-351 (2010).
5. S. Chung et al., Altered Relationship between Working Memory and Brain Microstructure after Mild Traumatic Brain Injury. AJNR Am J Neuroradiol 40, 1438-1444 (2019).
6. M. Murugavel et al., A longitudinal diffusion tensor imaging study assessing white matter fiber tracts after sports-related concussion. J Neurotrauma 31, 1860-1871 (2014).
7. M. A. Lancaster et al., Acute white matter changes following sport-related concussion: A serial diffusion tensor and diffusion kurtosis tensor imaging study. Hum Brain Mapp 10.1002/hbm.23278 (2016).
8. A. C. Duhaime et al., Magnetic resonance imaging studies of age-dependent responses to scaled focal brain injury in the piglet. J Neurosurg 99, 542-548 (2003).
9. L. L. Grate, J. A. Golden, P. J. Hoopes, J. V. Hunter, A. C. Duhaime, Traumatic brain injury in piglets of different ages: techniques for lesion analysis using histology and magnetic resonance imaging. J Neurosci Methods 123, 201-206 (2003).
10. J. C. Pareja, K. Keeley, A. C. Duhaime, C. P. Dodge, Modeling Pediatric Brain Trauma: Piglet Model of Controlled Cortical Impact. Methods Mol Biol 1462, 345-356 (2016).
11. J. Dobbing, J. Sands, Comparative aspects of the brain growth spurt. Early Hum Dev 3, 79-83 (1979).
12. S. H. Friess et al., Neurobehavioral functional deficits following closed head injury in the neonatal pig. Exp Neurol 204, 234-243 (2007).
13. M. Karlsson et al., Evaluation of Diffusion Tensor Imaging and Fluid Based Biomarkers in a Large Animal Trial of Cyclosporine in Focal Traumatic Brain Injury. J Neurotrauma 10.1089/neu.2020.7317 (2021).
14. L. S. Atlan, S. S. Margulies, Frequency-Dependent Changes in Resting State Electroencephalogram Functional Networks after Traumatic Brain Injury in Piglets. J Neurotrauma 36, 2558-2578 (2019).
15. H. A. Kinder et al., Traumatic Brain Injury Results in Dynamic Brain Structure Changes Leading to Acute and Chronic Motor Function Deficits in a Pediatric Piglet Model. J Neurotrauma 36, 2930-2942 (2019).
16. A. H. Netzley et al., Multimodal characterization of Yucatan minipig behavior and physiology through maturation Scientific Reports doi: https://doi.org/10.1101/2021.03.18.436053 (2021).
17. M. H. Prasad P, The position of the United States delegation to the ISO Working Group 6 on the use of HIC in the automotive environment. SAE transactions 106 (1985).
18. W. L. Pierpaoli C, Irfanoglu O, Barnett A, Basser P, Chang C, Koay C, Pajevic S, Rohde G, Sarlls J, Wu M (2010) TORTOISE: an integrated software package for processing of diffusion MRI data. in ISMRM.
19. H. Jiang, P. C. van Zijl, J. Kim, G. D. Pearlson, S. Mori, DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 81, 106-116 (2006).
20. G. Simchick et al., Pig Brains Have Homologous Resting-State Networks with Human Brains. Brain Connect 9, 566-579 (2019).
Figures