2331
Open-source modular 3D printed platform for in-vivo MRI experiments in awake mice and anesthetized mice and rats1Radiology, NYU School of Medicine, NEW YORK, NY, United States, 2Neuroscience Institute, NYU School of Medicine, New york, NY, United States, 3NYU School of Medicine, New York, NY, United States, 4Neuroscience Institute, NYU School of Medicine, NEW YORK, NY, United States
Synopsis
Despite their costly nature, the inexactitude of design in off-the-shelf MRI animal holders translates to a fastidious setup, iso-center misalignment, and incorrect animal body positioning. Our open-source 3D-print design provides precise iso-center alignment and minimizes motion artifact using its various head fixing mechanisms. It ensures comfortable positioning for rats and mice during in-vivo anesthetized and awake animal scan acquisition. The modular holder parts enable the same design to be used with other imaging modalities with minimal intervention. The design is low cost, ensures reproducibility and time efficiency of setup.
Introduction:
Efficient solutions for animal conditioning suitable for well-controlled and repetitive MRI experimentation are limited in number, costly and most of the time lack flexibility. With recent advances, affordable 3D printers have expanded the capability of the research community to design, prototype, and apply parts and systems that best suit their experiments1,2. Here, we introduce an open-source modular 3D-printable rodent conditioning fixture with a precise and adjustable head-fixing system for anesthetized and awake rodents.Method/results:
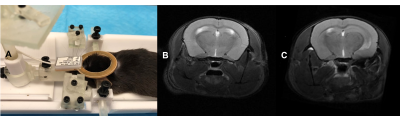
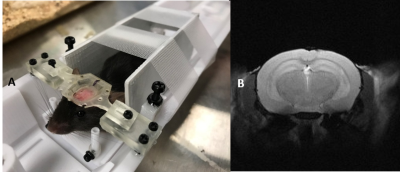
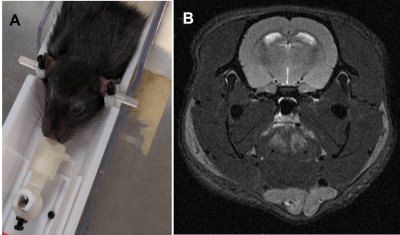
The proposed holder was designed using Fusion 3603. Small parts are made with resin-based printer (Form2, Formlabs) to achieve desired resolution. Larger parts were printed using Tough PLA material (Replicator-Z18, MakerBot). Our holder accommodates both anesthetized and awake rodents. Its base has an embedded ear-bar guiding mechanism for head-fixation of anesthetized mice and rats. The ear-bar system enables the user to achieve a fixed, precise, and repeatable head positioning while continuously delivering anesthetic agent. The adjustable bed platform enables proper body positioning and physiological comfort to the mouse. The base can also host a pair of removable stands for head fixation of awake mice. These stands can support matching fixtures formerly attached to animals’ skulls in a stereotaxic surgery. The holder design along with our extensive experience in training and employing head-fixed mice in neuroscientific experiments4 allows us to image awake animals. The flexible positioning of the isoflurane delivery mechanism, alongside with an interchangeable tooth bar sub-system, allows the utilization of the same setup for both mouse and rat with minimal intervention. The tooth-bar stand can be extended by an add-on that permits attachment of customizable holders for experiments requiring fiber optics, cables, etc. In awake mice experiments the nose-cone system can also be used for odor stimulation. Other add-on parts include a mouse bed platform, and heating covers. The base of the holder can be attached to various imaging modalities (e.g. MRI, CT, etc) with minor adjustments. In addition, the awake mice head-fixation system can be used in several neuroscientific experiments such as calcium imaging. These characteristics facilitate the design and execution of multi-modal investigations. Tapping 3D-printed plastic materials often comprises the quality of screw connections and can wear off in a short time. To avoid such quality issues and their dependency on printing technology, a tap-free approach was applied to assemble different parts of the system using nylon nuts (fixed to the 3D printed parts by UV cured superglue, Loctite) to secure the screws. For mouse imaging in both anesthetized and awake subjects, a birdcage RF-coil (ID=86-mm) was used in transmit-only for the excitation pulse and a single receive-only loop surface coil (ID=10mm) for the signal acquisition. A T2-weighted RARE sequence was used in both cases to test the stability of the setup using the following pulse parameters: TR=2500-ms, TE=33-ms, Turbo-factor=8, FOV=20-mm x 20-mm, Matrix size=256 x 256 resulting into 78-µm x 78-µm in-plane resolution with a total of 19 slices of 800-µm thickness. Scans were repeated over 10 times resulting into an imaging time of 13-min. While repetitions were averaged online in anesthetized mice, individual repetitions were acquired separately for the awake mouse. The corresponding image sets were subsequently realigned retrospectively using ImageJ (NIH) prior to averaging to account for erratic motions of the awake mouse. For anesthetized rat imaging, the birdcage volume coil (ID=86mm) was used in Tx/Rx to acquire the same RARE sequence under identical parameters and imaging time as in mouse experiments but with a FOV = 35-mm x 35-mm resulting into 137-µm x 137-µm in-plane resolution We performed preliminary experiments on brain imaging of both mice and rats to examine our 3D printed design. Figure 2.A illustrates the experimental setup for anesthetized mice. Vitals sign were monitored via a rectal probe for body temperature and an air cushion located underneath the abdomen to monitor pressure variations during respiration. The body temperature was controlled via a water-circulating heating cover on top of the mouse. Figure 2.B and C illustrate example T2 scans of two anesthetized mice with minimal adjustments in between, confirming the repeatability of scanning using our platform. Figure 3.A depicts the identical setup but this time configured for awake animal scanning. The awake animal, formerly trained for head-fixation, is placed on the base while its head is secured with a skull fixture coupled to matching pieces embedded in the holder. Side structures surrounding the animal help reduce its stress. Figure 3.B depicts the ability of this setup to perform motion-artifact-free MR imaging in awake mice. Figure 4.A shows a separate copy of the holder dedicated to experiments involving anesthetized rats. A formerly anesthetized rat was accommodated into the set-up in a similar way as for the mouse. A sample MR scan is illustrated in Figure 4.B.Discussions/Conclusions:
Our open-source modular design is easy-to-assemble, easy-to-use, and compatible with various animal MRI experiments. It can also be adapted to other imaging and recording modalities. Our preliminary experiments demonstrate the effectiveness of the set-up in awake and anesthetized animal conditioning for different image acquisition. Provided with open-source CAD design files5, our system is easy-to-modify and low-cost to build and can be tailored to the researcher’s need.Acknowledgements
“This work was supported, in part, by NSF-DMREF under Award Number DMR 1728858 and was also performed at the NYU Langone Health Preclinical Imaging Laboratory, a shared resource partially supported by the NIH/SIG 1S10OD018337-01, the Laura and Isaac Perlmutter Cancer Center Support Grant NIH/NCI P30CA016087 and the NIBIB Biomedical Technology Resource Center Grant NIH P41 EB017183”.References
[1] Fan et al., “Progressive 3D Printing Technology and Its Application in Medical Materials”, Fron. In Pharma., 2020, https://doi.org/10.3389/fphar.2020.00122[2] Vöröslakos et al., “Metal Microdrive and Head Cap System for Silicon Probe Recovery in Freely Moving Rodent.” ELife, 2021, elifesciences.org/articles/65859.
[3] https://www.autodesk.com/products/fusion-360/overview
[4] English et al., “Pyramidal Cell-Interneuron Circuit Architecture and Dynamics in Hippocampal Networks”, Neuron, 2017, https://doi.org/10.1016/j.neuron.2017.09.033
[5] https://github.com/zbenyouss/Modular-3D-printed-Platform-For-In-vivo-MRI
Figures
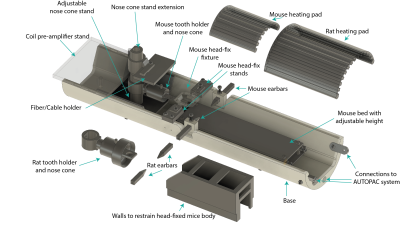
Figure 1. Open-source 3D printable modular holder design for in-vivo MRI experiment on anesthetized mice and rats and awake mice