2326
Preclinical MR spectroscopy of GABA: conventional PRESS versus spectral editing with MEGAPRESS and HERMES
Diana Rotaru1, Steve Sawiak2,3, Camilla Simmons1,4, Eugene Kim1,4, Maria Elisa Serrano Navacerrada1,4, Davide Di Censo1,4, Adrien Le Guennec5, Richard Edden6,7, David Lythgoe1, and Diana Cash1,4
1Neuroimaging, King's College London, London, United Kingdom, 2Behavioural and Clinical Neuroscience Institute, University of Cambridge, Cambridge, United Kingdom, 3Wolfson Brain Imaging Centre, University of Cambridge, Cambridge, United Kingdom, 4BRAIN Centre (Biomarker Research And Imaging for Neuroscience), King's College London, London, United Kingdom, 5NMR Facility, Wolfson CARD, Guy's Campus, King's College London, London, United Kingdom, 6Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, Baltimore, MD, United States, 7F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
1Neuroimaging, King's College London, London, United Kingdom, 2Behavioural and Clinical Neuroscience Institute, University of Cambridge, Cambridge, United Kingdom, 3Wolfson Brain Imaging Centre, University of Cambridge, Cambridge, United Kingdom, 4BRAIN Centre (Biomarker Research And Imaging for Neuroscience), King's College London, London, United Kingdom, 5NMR Facility, Wolfson CARD, Guy's Campus, King's College London, London, United Kingdom, 6Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, Baltimore, MD, United States, 7F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
In vivo GABA measurements with conventional (PRESS) and spectral editing methods (MEGAPRESS and HERMES) were investigated in healthy mice at 9.4 T. Animals were divided in two groups, control mice injected with saline and mice injected with Aminooxyacetic acid to intentionally elevate GABA concentration levels (n=7 per group). Our results show all three methods successfully discriminate GABA from overlapping metabolites with stronger signals. The precision of concentration estimates is more reliable for spectral editing methods given their specificity to GABA. These outcomes suggest spectral editing methods are less susceptible to systematic biases.
Introduction
Gamma-aminobutyric acid (GABA) is a key metabolite involved in inhibitory neurotransmission. Despite advancements in human GABA spectral editing, it is frequently assumed that the high B0 of preclinical scanners is sufficient to detect GABA without spectral editing. Often, in preclinical GABA MRS studies, conventional sequences (PRESS1) are used. Comparison between preclinical PRESS and spectral editing methods have not previously been reported. We compared MEGAPRESS2 and HERMES3 against PRESS and assessed their GABA discrimination efficiency.Methods
Data were acquired on a 9.4T Bruker BioSpec 94/20 MR scanner with ParaVision 6.0.1, using an 86-mm volume resonator and phased array mouse head receive-only coil. Fourteen mice (control and drug-treated mice, n=7 per group) and three in vitro samples containing GABA (Sigma Aldrich) concentrations of 2 mM, 10 mM, and 20 mM in phosphate-buffered saline were scanned. An increase in GABA concentration levels was induced using aminooxyacetic acid4 (AOAA). Animal work was in accordance with the Animals (Scientific Procedures) Act 1986, under standard laboratory conditions. Four hours prior to their spectroscopy acquisition, animals were dosed with saline (10ml/kg i.p.) or AOAA, (100mg/kg i.p). Initially, mice were anesthetized with isoflurane (5%) in oxygen/air (4/10) and then switched to subcutaneous medetomidine bolus (0.05mg/kg) followed by medetomidine infusion (0.1mg/kg/h)) with 0.4% isoflurane. Internal body temperature (37±0.5o C) and breathing rate (~70-150 breaths/minute) were continuously monitored and maintained with a rectal temperature probe, heated pad, and thoracic respiratory sensor.The MR acquisition protocol consisted of localizers, structural scans and three spectroscopy acquisitions (PRESS, MEGAPRESS, HERMES). The spectroscopy voxel was placed in the phantom centre (3x3x3mm3) or over bilateral striata (2x1.5x6mm3, Dorsal/Ventral x Rostral/Caudal x Right/Left) (Figure 1). B0-map and localized shimming and frequency adjustments were applied before data acquisition. PRESS, MEGAPRESS and HERMES acquisition parameters were: TR 2s; TE 8.61/68/80ms; 4.4kHz bandwidth; 4096 data points; 640 averages per acquisition; working frequency 2.7ppm; VAPOR water suppression. MEGAPRESS and HERMES editing pulses were created using a template pulse shape (8.5ms duration, 200Hz bandwidth, 180° flip angle) with additional cosine modulation for dual-band pulses. Editing frequencies were 1.9ppm (GABA), 4.56ppm (GSH) and 7.5ppm (the non-edited condition). Acquisition time per sequence was 21’20”. Mean (SD) in vivo water linewidths were 17.73 (2.38) Hz.
Data were imported into Matlab for pre-processing with a custom-written pipeline based on FID-A5 scripts and functions. PRESS and spectrally edited data underwent conversion to complex data, digital filter delay correction, fast-decaying exponential filtering, receiver-coil combination, removal of motion corrupted averages, frequency and phase correction, and spectral registration6. PRESS and Hadamard-reconstructed GABA difference spectra were saved to RAW LCModel7 compatible format. The unsuppressed water data was recovered from the method file. Spectra were analyzed with LCModel (6.3-1L). For each set of acquisition parameters (TR, TE, RF pulses waveforms, pulse sequence timings, etc.) and in vivo/in vitro scanning, basis sets were simulated for 813 spatial positions and control parameters were created.
The relationship between true and LCModel-detected GABA concentrations was assessed by Pearson correlation. GraphPad Prism (version 9.2.0) two-way ANOVA analysis with Šídák's multiple comparisons tests were employed to determine significant differences between mouse groups for all methods. For between-method comparison, the intraclass correlation coefficient (ICC) and 95% Confidence Interval (CI) were estimated using SPSS (version 27.0.1.0), and Bland-Altman plots with GraphPad Prism.
Results
Correlation results for phantom MRS concentrations versus true GABA concentrations are illustrated in Figure 2. Figure 3 shows AOAA-treated mouse example spectra. Figure 4.a displays mean and SD plots of GABA concentrations (i.u.) and Cramér–Rao lower bound error estimates (CRLBs,%), while Figure 4.b shows significantly higher GABA concentrations in AOAA-treated mice than in controls for all methods (p<0.001). The ICC (95% CI) was 0.938 (0.029–.982). Bland-Altman plots are displayed in Figure 5.Discussion
Phantom correlation results show excellent agreement between LCModel estimated and true concentrations for all three methods. Distribution/violin plots display a greater spread in AOAA concentrations, especially for PRESS, while the ANOVA results show all methods successfully discriminate between the two groups. Considering MEGAPRESS (without macromolecule suppression) as the gold standard, these results also indicate PRESS leads to an overestimation of GABA levels. The high ICC implies consistency between methods, but Bland-Altman plots indicate systematic differences between PRESS and spectral editing techniques (PRESS data points above the average difference). One potential explanation for these differences is the incorrect fitting of the GABA basis function by LCModel in the presence of confounding spectral peaks. Despite using sequence-matched simulated basis sets and disabling LCModel automated corrections, there might be LCModel hidden control parameters not fully optimized for field strength, data type, or pulse sequence parameters. Although group differences were detected by all methods, spectral editing comes with the advantage of a metabolite-tailored data acquisition, where macromolecules and lipids contribution is reduced through sub-spectra subtraction. Future work includes the comparison of MRS results with NMR measurements of extracted brain tissue.Conclusions
PRESS and spectral editing methods (MEGAPRESS and HERMES) were compared at 9.4T for the first time. All methods successfully distinguished between the treatment groups, but PRESS appears less precise and more prone to systematic bias. PRESS GABA estimates may be sufficient when additional metabolites and group differences are more important than absolute quantification, otherwise, spectral editing should be used.Acknowledgements
No acknowledgement found.References
1. Bottomley PA, Ann N Y Acad Sci, 1987, 508, 333–348. 2. Mescher M, & Garwood M, NMR Biomed, 1998,11, 266–272. 3. Chan K. et al, Magn Reson Med, 2016, 76, 11–19. 4. Löscher W, Hörstermann D, Naunyn-Schmiedeberg's Arch Pharmacol, 1994, 349, 270–278. 5. Simpson R et al, Magn Reson Med, 2017, 77, 23–33. 6. Near J, Magn Reson med, 2014, 73, 44-50. 7. Provencher S, Magn Reson Med, 1993, 30, 672–679.Figures
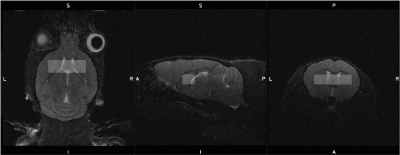
Figure 1. Mouse MRS voxel placement over the striatum, partially overlapping the prefrontal cortex and ventricles. The voxel was sized 2 x 1.5 x 6 mm3 (Dorsal/Ventral x Rostral/Caudal x Right/Left)
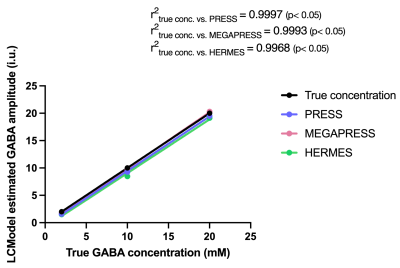
Figure 2. Phantom correlation results indicate a high positive correlation between GABA concentrations determined by PRESS (blue), MEGAPRESS (red) and HERMES (green) when compared to true GABA concentration (r2 = 0.9997, 0.9993, 0.996 and p<0.05 for all). LCModel concentrations were not corrected for relaxation times.
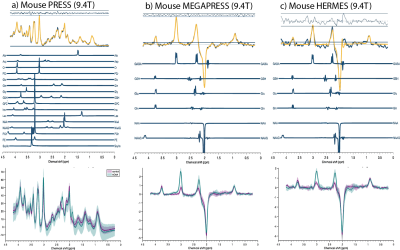
Figure 3. Top row: PRESS (a), MEGAPRESS (b) and HERMES (c) MRS sample spectra from one AOAA-treated mouse. Overlayed data (dark blue) and fit (yellow) are presented with corresponding residuals above and basis sets below. Bottom row: mean and standard deviation plots of all analyzed datasets divided into control (purple) and AOAA (green) groups for each method.
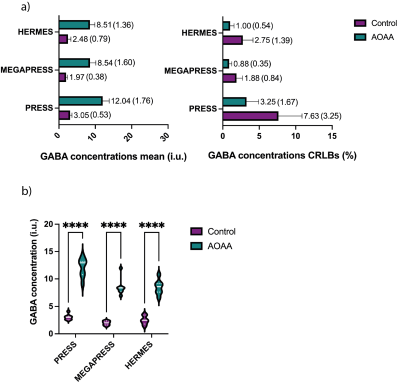
Figure 4. a) Mean and SD plots for control and AOAA-treated mice for PRESS, MEGAPRESS and HERMES, and b) 2-way ANOVA results showing GABA concentrations in AOAA-treated mice are significantly higher than in control mice (p<0.0001) for all methods. LCModel concentrations were not corrected for relaxation times.
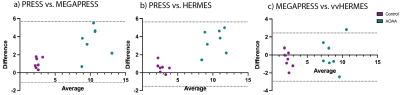
Figure 5. Bland-Altman plots for (a) PRESS vs. MEGAPRESS, (b) PRESS vs. HERMES, (c) MEGAPRESS vs. HERMES. Purple dots depict control mouse concentrations, while green ones correspond to AOAA-treated mice. LCModel concentrations were not corrected for relaxation times.
DOI: https://doi.org/10.58530/2022/2326