2325
Animal models using C57BL/6J mice are at risk of data misinterpretation due to random occurrences of animals with high levels of brain glutamine1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Department of Pediatrics, University of Minnesota, Minneapolis, MN, United States
Synopsis
Wild-type and transgenic mice derived from the C57BL/6J mouse strain are widely used in animal models of different human diseases. However, this mouse strain suffers from random appearances of sporadic congenital portosystemic shunts resulting in extremely high glutamine levels in the brain. In our recent study aimed at effects of maternal obesity on their offspring we found that in 9 out of 31 studied C57BL/6J mice had high levels of brain Gln. The purpose of this abstract is to highlight the awareness of this persisting problem in animal models based on C57BL/6J background strain.
INTRODUCTION
We reported for the first time at the ISMRM Meeting in Montreal, a decade ago, extremely high levels of brain glutamine (Gln) in a cohort of C57BL/6J 1. These high Gln mice appeared only in the C57BL/6J strain and occurred randomly in wild-type and experimental (transgenic Huntington mice) cohorts. This puzzling problem was later explained as a consequence of sporadic congenital portosystemic shunts bypassing the liver in C57BL/6J mice 2. Suboptimal hepatic function results in increased levels of ammonia in the blood, which can cross the blood-brain barrier into the brain. The brain avoids ammonia toxicity by converting glutamate to Gln. This effect is similar to hepatic encephalopathy, resulting in increased Gln and decreased myo-inositol (osmolyte) in the brain. In our recent study aimed to investigate the hypothalamic neurochemical profile in adult C57Bl/6 mice exposed to in utero obesogenic diet we found that 9 out of 31 (29%) mice had high levels of brain Gln. The objective of this abstract is to highlight this persisting problem in animal models based on C57BL/6J strain.METHODS
Spontaneously breathing adult mice were anesthetized with 1.0 – 1.5% isoflurane. The body temperature was maintained at 37oC. All MRI/MRS data were measured using the 9.4T magnet interfaced to (Varian/Agilent) console. Multislice FSE technique was used for imaging (slice thickness = 0.8 mm, ETL = 8, ESP = 12 ms). In vivo 1H MRS data were acquired from the region of hypothalamus using FASTMAP B0 shimming 3 and STEAM 4 (TE = 2 ms, TR = 5 s) or LASER 5 localization sequence (TE = 15 ms, TR = 5 s) combined with VAPOR water suppression 4. Metabolites were quantified using LCModel with the spectrum of fast relaxing macromolecules included in the basis set. Unsuppressed water signal was used as an internal reference.RESULTS
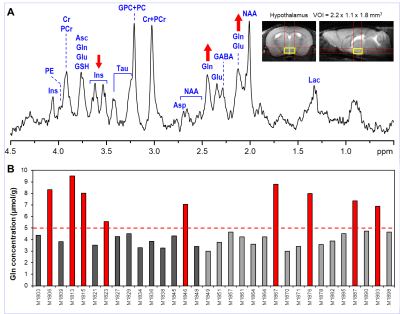
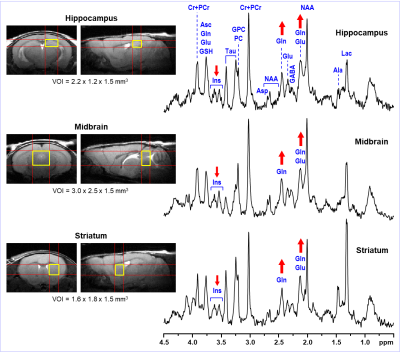
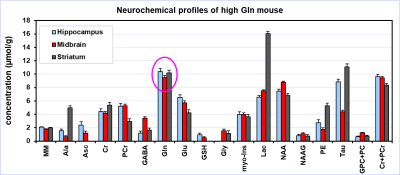
The typical spectral pattern in high Gln mice is shown in Fig. 1. The bar diagram shows the Gln levels found in 31 scanned C57BL/6J mice. All 9 high Gln animals had to be excluded from the final analysis. Over the years, we have observed these high Gln in different animal models based on C57BL/6J strain (e.g., spinocerebellar ataxia 6 or animal studies focused on safety at ultra-high magnetic fields 7. High Gln levels are not localized to a specific brain region in the these mice, but affect the whole brain as is shown in Fig. 2. Neurochemical profiles of the high Gln mouse acquired from the hippocampus, midbrain and striatum are considerably different from each other (Fig. 3), but the Gln concentration is almost the same (~10 µmol/g) in all three brain regions.DISCUSSION
Random occurrence of high Gln mice in wild-type or genetically modified C57BL/6J strain is a persistent and serious problem and could lead to misinterpretation of acquired data. Use of high Gln mice may lead to false positive diagnosis of conditions associated with high Gln, such as neurodegenerative disorders. Despite reports of this problem, this mouse strain continues to be widely used in preclinical research. 1H MRS is a unique technique that can identify this problem relatively easily and non-invasively. Neuroscience studies that do not include 1H MRS are at risk of inclusion of high Gln animals and resultant bias in data interpretation.CONCLUSION
Animal models using wild-type or genetically modified mice derived from C57BL/6J mouse strain are at risk of data misinterpretation because of random occurrences of animals with extremely high levels of bran Gln.Acknowledgements
Supported by: NIH grants P41 EB027061, P30 NS076408, K12 BIRCWH and Department of Pediatrics R AwardsReferences
1. Tkac I, Zacharoff L, Dubinsky JM. Longitudinal study of neurochemical changes in Q140 mouse model of Huntington’s disease. Annual ISMRM Meeting, Montreal, Canada. 2011.
2. Cudalbu C, McLin VA, Lei H, et al. The C57BL/6J mouse exhibits sporadic congenital portosystemic shunts. PLoS One. 2013;8(7):e69782.
3. Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43(2):319-323.
4. Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41(4):649-656.
5. Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153(2):155-177.
6. Emir UE, Brent Clark H, Vollmers ML, Eberly LE, Oz G. Non-invasive detection of neurochemical changes prior to overt pathology in a mouse model of spinocerebellar ataxia type 1. J Neurochem. 2013;127(5):660-668.
7. Tkac I, Benneyworth MA, Nichols-Meade T, et al. Long-term behavioral effects observed in mice chronically exposed to static ultra-high magnetic fields. Magn Reson Med. 2021;86(3):1544-1559.
Figures