2324
Correlation methods between X- ray phase contrast imaging, MRI and histology for the study of the nervous central system1CNR -Institute of Nanotechnology, Lecce Unit & Roma Unit, LECCE, Italy, 2URPhyM – NARILIS, Université de Namur, Namur, Belgium, 3Museo Storico della Fisica e Centro Studi e Ricerche Enrico Fermi, Rome, Italy, 4NEUR division, Université catholique de Louvain (UCLouvain), Institute of NeuroScience (IoNS), Brussels, Belgium, 5Department of Pharmacy and Biotechnology, University of Bologna, Bologna, Italy, 6A. I. Virtanen Institute for Molecular Sciences, University of Eastern Finland, Kuopio, Finland, 7CNR -Institute of Nanotechnology, Lecce Unit, LECCE, Italy, 8CNR -Institute of Nanotechnology, Roma Unit, Rome, Italy
Synopsis
Many serious pathologies of the central nervous system (CNS) are related to anomalous development or damages to the vascular and neuronal networks. Here, we show our approach based on MRI/XPCT, XPCT/ HISTOLOGY combination in order to study neuronal and vascular alterations following a damage in mouse models.
INTRODUCTION
Many serious pathologies of the central nervous system (CNS) are related to anomalous development or damages to the vascular and neuronal networks [1,2]. A detailed structural characterization reveals useful even when we want to improve our knowledge of pathological processes effects following a neurological disease as well as an injury. Improvements in biological tissue characterization in pathological conditions can be obtained from approaches based on integrating structural information arising from different experimental techniques. X ray phase contrast micro tomography (XPCT) is a very attractive tool for the study of pathological conditions, as it allows visualizing weakly absorbing structures and provides 3D stacks of images of vessels, cells and axons at the micron scale. We have recently demonstrated the capability of XPCT to visualize simultaneously the architecture of vascular and neuronal networks in the mouse spinal cord, down to a hundred of nanometers-resolution, without contrast agent or tissue sectioning [3, 4, 5]. Moreover, interesting improvements can be achieved by combining XPCT images with MRI images [4] and histology. In this work, we show our approach based on co-registration methods between ex vivo diffusion tensor imaging (DTI) and ex vivo XPCT of mouse brain and spinal cord. In addition, we show a XPCT/ histology co-registration method that we used to analyse the phrenic motoneurons(PhMNs) loss following a spinal cord injury(SCI).METHODS
This study includes C57BL/6J healthy mouse brain and a C57BL/6J contralaterally SCI mouse.DTI acquisition and processing: Ex vivo DTI was acquired with a 9.4 T scanner using segmented spin-echo EPI (TE = 32 ms, TR = 1 s, b-value = 3000 s/mm, 42 diffusion directions, and 125 μm-isotropic resolution) and used to generate fractional anisotropy (FA) and directionally encoded colored maps.
XPCT imaging: Ex vivo XPCT data were acquired at ID17 beamline at the European Synchrotron Radiation Facility (ESRF) in Grenoble, France and at TOMCAT beamline of Paul Scherrer Institut (PSI). We used free-space propagation method, with beam energy = 34 keV, CCD pixel size = 3 μm, detector-sample distance ≈ 2.3 m (ID 17) and while beam energy = 17 keV, CCD pixel size = 1,6, 0,64 μm, detector-sample distance ≈ 5 cm (TOMCAT).
Histological tracer: Retrograde anatomical tracing procedure was used for specific visualization of the PhMNs column via retrograde transport of fluorescent tracer cholera toxin β subunit (CTβ). The procedure was done at University of Namur.
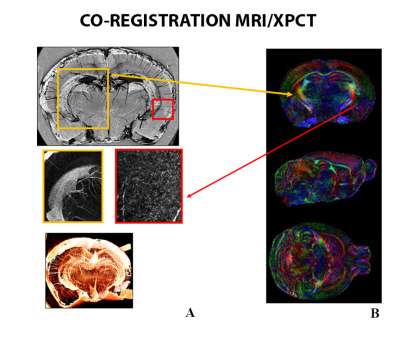
Registration between MRI and XPCT: XPCT images were low pass filtered to avoid aliasing, and down-sampled in order to roughly match the dMRI resolution. Fractional anisotropy(FA) images were then coregistered to the corresponding XPCT using advanced normalization tools (ANTs).
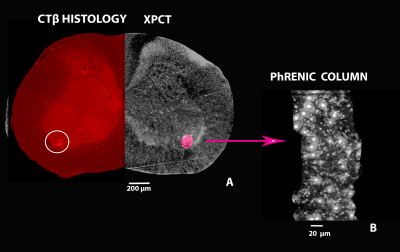
Registration between XPCT images and histological sections: Each registration was repeated five times: three using different cost functions (correlation ratio, mutual information and normalized mutual information) and two, imposing a voxel-wise weighting on the area of phrenic column, to affect the correlation ratio cost function. Both XPCT and histological images were previously semi-automatically segmented using an edge classification algorithm and the area outside the spinal cord was masked out. Histological sections were resampled to match the XPCT images (trilinear interpolation). We manually draw binary ROIs corresponding to the phrenic column on the histological sections and transposed on the tomographic space using the 5 geometric transformations aforementioned. The five registered ROIs were then summed up.
PhMNs quantification: PhMNs were then quantified by applying filtering procedures based on PhMNs size and by running the analysis in two VOIs (136×166×350 µm3), corresponding to the projected phrenic columns.
RESULTS
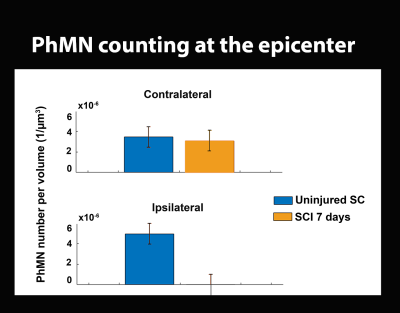
dMRI/XPCT co-registration of the healthy mouse brain was done exploiting the perfect contrast match between Fractional Anisotropy maps and XPCT in the corpus callosum (Fig. 1).Based on the co-registered ROIs in individual slices, a 3D volume has been generated to subsequently allow for PhMNs counting within the phrenic column at C4 spinal level. The localization of the the rostro-caudal arrangement of the phrenic column can be seen in Fig. 2 A, together with the chain-like arrangement of MNs inside those phrenic columns (Fig. 2 B). We analyzed the SCI-induced cell death at 7 days post SCI (Fig. 3) and found that phrenic MNs were not detectable anymore with XPCT imaging at 7 days post-SCI. In comparison with an uninjured spinal cord, only 2% of PhMNs in the ipsilateral phrenic column were spared, whereas, in the contralateral side, there was a non-significant decrease (Fig. 3).
DISCUSSION
In this work, we have demonstrated that complementary information can be integrated and used as a new powerful tool for preclinical studies. Specifically, we were able to co-register dMRI to XPCT thus demonstrating the possibility to increase DTI resolution as well as to co-register histological sections to XPCT thus allowing to quantify phrenic neurons loss following a contralateral injury [6].CONCLUSION
In conclusion, our approach enhances the specificity of the information which is especially useful when we are interested in vascular and neuronal damage and paves the way for the increase of standard imaging techniques (MRI) resolution.Acknowledgements
The FISR Project “Tecnopolo di nanotecnologia e fotonica per la medicina di precisione” (funded by MIUR/CNR, CUP B83B17000010001), the TECNOMED project(funded by Regione Puglia, CUP B84I18000540002) is acknowledged for financial support, the Italian Ministry of Health Young Researcher Grant 2013 (GR-2013-02358177).The COST Action CA16122 “Biomaterials and advanced physical techniques for regenerative cardiology and neurology is acknowledged for networking support.References
[1] J. J.,Kril, et al. "Patients with vascular dementia due to microvascular pathology have significant hippocampal neuronal loss.." Journal of Neurology, Neurosurgery & Psychiatry, ,2002, 72.6, 747-751.
[2]S Chen, J.D Pickard, N.G Harris, "Time course of cellular pathology after controlled cortical impact injury", Experimental Neurology, 2003, 82, 87-102,
[3] M. Fratini, I. Bukreeva, et al., “ Simultaneous submicrometric 3D imaging of the micro-vascular network and the neuronal system in a mouse spinal cord”, Scientific reports, 2015, 5, 8514.
[4]M. Fratini, A. Abdollahzadeh, M. DiNuzzo, R.A. Salo, L. Maugeri, A. Cedola, F. Giove, O. Gröhn, J. Tohka, A. Sierra, Front. neurosci, 2020, 14.
[5] E. Stefanutti., A. Sierra, P. Miocchi, L. Massimi, F. Brun,L Maugeri, et al. "Assessment of the effects of different sample perfusion procedures on phase-contrast tomographic images of mouse spinal cord. " J. Instrum. 13, 2018.:C03027.
[6] Nicaise, C., Frank, D.M., Hala, T.J., Authelet, M., Pochet, R., Adriaens, D., Brion, J.-P., Wright, M.C. and Lepore, A.C. "Early phrenic motor neuron loss and transient respiratory abnormalities after unilateral cervical spinal cord contusion." Journal of neurotrauma, 2013, 30, 1092-1099.
Figures