2321
Feasibility of Cardiovascular Magnetic Resonance Imaging of In Vivo Mouse Heart using Multitasking at 9.4T1Wuhan United Imaging Life Science Instrument Co., Ltd., Wuhan, China, 2UIH America, Inc., Houston, TX, United States
Synopsis
The feasibility of cardiac T1 mapping in mouse is demonstrated at 9.4T using Multitasking, an ECG-free and free-breathing technique. High spatial- and temporal-resolution T1 maps of healthy mouse hearts without either respiratory or cardiac triggering were obtained. This technique simplifies experimental setup and opens possibilities of investigating numerous heart disease/transgenic mouse models.
Introduction
The use of rodent models facilitates preclinical research in human heart diseases such as myocardial infarction and cardiomyopathy [1,2]. Among others, mouse becomes popular in preclinical myocardial disease studies since mouse models require the shortest development period, have the largest number of genetically-modified/disease models and the lowest cost in mammals.Cardiac Magnetic Resonance (CMR), including scans such as morphology, function, perfusion, delayed enhancement and parametric mapping [3,4], provides comprehensive evaluation of the heart. Despite T1 mapping being a promising CMR tool to study myocardial function and physiology, it is challenged at high respiratory and heart rates. Previous studies aiming at T1 mapping in rodent models relied on ECG signal that is often poor at high field strength, potentially leading to image blurring and inaccuracies in quantification [5,6]. CMR Multitasking is an emerging technique that can resolve multiple ‘tasks’ simultaneously using a low-rank tensor model [7,8], with significantly reduced data acquisition time. Without breath-holding or the use of ECG, multitasking can produce high spatial- and temporal- resolution cardiac-phase-resolved myocardial T1 maps. Notably, a recent study demonstrated its feasibility in the imaging of a rat heart failure model [9].
Compared to rat, imaging of mouse heart is more challenging due to its reduced heart size, higher respiratory (e.g. 80-230 bpm) and heart rates (e.g. 310-840 bpm). The feasibility of T1 mapping using Multitasking in mouse at 9.4T is investigated in this study.
Methods
Animals: Seven C57BL/6 adult mice (8-week old, male, 24.7-26g) were obtained from a nearby SPF facility and received 0.1mL 10% chloral hydrate intraperitoneally. Once anesthetized, mice were carefully placed in a tail-prone position and body temperature was maintained via circulating warm water. Experiments followed local animal experimentation guidelines.MRI scan and analysis: MR experiments were performed in a horizontal 30cm-inner-diameter 9.4T magnet (uMR 9.4T, United Imaging Healthcare, China) with a gradient insert having 1000mT/m strength and 9000T/m/s slew rate. A 2-channel volume coil was used for transmitting and a 4-channel surface coil for receiving.
After localizers, 3D shimming using gradient-echo was performed around the mouse heart. A 2D T1 cine Multitasking sequence was developed following a previous application [9], and the scan parameters were adjusted for mouse imaging. Parameters were: FOV=32mm*32mm, matrix=208*208, slice thickness = 1mm, flip angle =5°, TE/TR=2.14/4.6ms, bandwidth=350 Hz/pixel. A total of 200 inversion recovery modules were used with 630 radial spokes following each inversion recovery module. The scan time was 580 s for each slice. Three axial slices were scanned on each mouse to cover the entire heart. To ensure minimal mouse position change throughout the experiments, 2D radial GRE images were also acquired before and after Multitasking scan, for reference of anatomies.
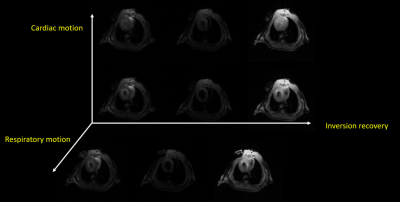
Offline reconstruction was performed with 4 respiratory and 10 cardiac states, following Multitasking reconstruction framework. Cutoff frequencies for respiratory and cardiac binning used in machine learning method was adjusted to reflect mouse physiology. Finally, T1 value at each pixel is independently fitted using least-squares fitting. Output of the prototype application included respiratory-motion-resolved image series, cardiac-motion-resolved image series, inversion recovery image series, and T1 cine maps. The averaged T1 values of the entire myocardium in a diastolic and systolic phase from each slice was measured by drawing a region-of-interest (ROI) by an experienced researcher. Numerical values were presented as mean ± standard deviation.
Results
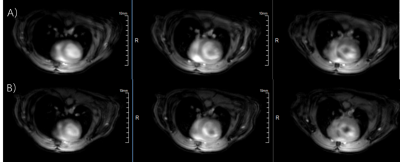
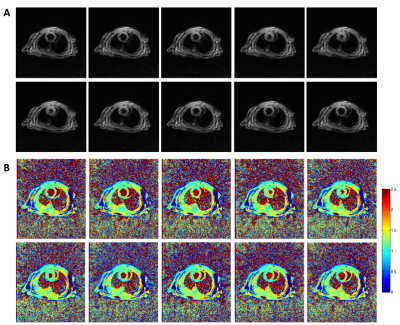
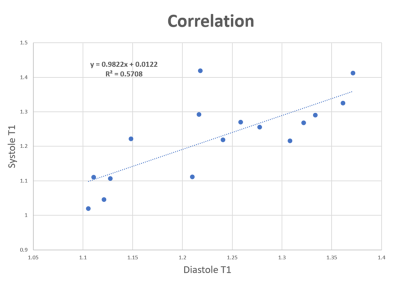
Figure 1 shows typical radial GRE reference images before (Fig 1A) and after (Fig 1B) Multitasking scan. After examining major anatomical structures of the chest (e.g. heart and spinal cord) for obvious bulk motion, one mouse was excluded. 16 of the remaining 18 slices had heart coverage and thus was used for analysis. Figure 2 shows typical mouse Multitasking images along the respiratory, cardiac, and inversion recovery dimensions. Figure 3 displays typical T1 maps across all cardiac phases. The average myocardium T1 values at diastole and systole were 1.23 ± 0.09 s and 1.22 ± 0.12 s, respectively. There is strong correlation between T1 values at diastole and systole (R=0.76, Figure 4) but no statistical difference between them (p = 0.62).Conclusion and Discussion
The feasibility of in vivo T1 mapping of mouse heart at 9.4T using CMR T1 Multitasking was demonstrated. The measured T1 values are generally in line with those previously published [5,9]. Multitasking is a promising tool for preclinical research involving mice, without requiring respiratory gating or cardiac triggering. This opens the possibilities in the study of various disease models such as myocardial infarction, hypertrophy, and heart failure.Acknowledgements
This work was partially facilitated by a non-exclusive license agreement between Cedars-Sinai Medical Center and United Imaging Healthcare.References
[1] Blankesteijn WM, Creemers E, Lutgens E, Cleutjens JPM, Daemen M, Smits JFM. Dynamics of cardiac wound healing following myocardial infarction: observations in genetically altered mice. Acta Physiol. Scand. 2001; 173: 75–82.
[2] Lim DS, Lutucuta S, Bachireddy P, Youker K, Evans A, Entman M, Roberts R, Marian AJ. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation 2001; 103: 789–791.
[3] Addy NO, Wu HH, Nishimura DG. A Simple Method for MR Gradient System Characterization and k-Space Trajectory Estimation. Magn Reson Med. 2012 Jul; 68(1): 120–129.
[4] Campbell-Washburn AE, Xue H, Lederman RJ, et al. Real-time Distortion Correction of Spiral and Echo Planar Images Using the Gradient System Impulse Response Function. Magn Reson Med 2016 Jun 01;75(6):2278-85.
[5] Coolen BF, Geelen T, Paulis LE, Nauerth A, Nicolay K, Strijkers GJ. Three dimensional T1 mapping of the mouse heart using variable fip angle steady-state MR imaging. NMR Biomed. 2011;24(2):154–62.
[6] Smit H, Guridi RP, Guenoun J, Poot DH, Doeswijk GN, Milanesi M, Bernsen MR, Krestin GP, Klein S, Kotek G. T1 mapping in the rat myocardium at 7 tesla using a modifed CINE inversion recovery sequence. J Magn Reson Imaging. 2014;39(4):901–10
[7] Shaw JL, Yang Q, Zhou Z, Deng Z, Nguyen C, Li D, Christodoulou AG. Free-breathing, non-ECG, continuous myocardial T1 mapping with cardiovascular magnetic resonance multitasking. Magn Reson Med. 2019 Apr;81(4):2450-2463.
[8] Christodoulou AG, Shaw JL, Nguyen C, Yang Q, Xie Y, Wang N, Li D. Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nat Biomed Eng. 2018;2(4):215.
[9] Han P, Zhang R, Wagner S, Xie Y, Cingolani E, Marban E, Christodoulou AG, Li D. Electrocardiogram-less, free-breathing myocardial extracellular volume fraction mapping in small animals at high heart rates using motion-resolved cardiovascular magnetic reesonance multitasking: a feasibility study in a heart failure with preserved ejection fraction rat model. J Cardiovasc Magn Reson. 2021 Feb 11;23(1):8
Figures