2315
Measuring brain temperature changes in the rhesus monkey maintained under isoflurane anesthesia using diffusion MRI1Yerkes National Primate Research Center, Emory University, Atlanta, GA, United States
Synopsis
Therapeutic hypothermia can improve neurological recovery and reduce mortality in acute stroke. Pharmacologically-induced hypothermia (PIH) has been investigated in preclinical and clinical studies. Usually the core temperature (rectal or esophageal) is measured to monitor the temperature changes of the subject during the treatment. However, it remains unknown how the brain temperature is affected by PIH as the brain temperature can be different from the core temperature and affected by the anesthesia. In the present study, the brain temperature changes of adult rhesus monkeys maintained under isoflurane for over 3 hours were examined and evaluated using diffusion MRI.
Introduction
Therapeutic hypothermia has been demonstrated to improve neurological recovery and reduce mortality in patients of acute stroke or traumatic brain injury (TBI) and several approaches including pharmacologically-induced hypothermia (PIH) are investigated in preclinical and clinical studies [1]. Usually the core temperature (rectal or esophageal) is monitored to measure the temperature changes of the subjects during the treatment. However, it remains unknown how the brain temperature is affected quantitatively by the hypothermia because the brain temperature can be different from the core temperature and affected by the anesthesia which is generally used in neuroimaging examination of animal models. In the present study, the brain temperature changes of adult rhesus monkeys maintained under isoflurane for over 3 hours were examined and evaluated using diffusion MRI (dMRI) thermometry.Methods
Water phantom was used to evaluate the dMRI thermometry with the temperature changes of ~1 and ~2.5 °C. Diffusion MRI was performed on a 3T clinical scanner using the following parameters: TR/TE =4000ms/93ms, voxel site: 1.0 x 1.0 x 1.0 mm3, 30 gradient directions with the b values = 400, 1000 s/mm2. Healthy adult monkeys (n=4, 11-15 years old, 8-10kg) were used in the study and scanned for over 3 hours (195-220 minutes) with the same protocol and scanner. The animals’ core temperatures were maintained for > 30 minutes after the animal was placed in the scanner to ensure that the animals’ physiology was stable before dMRI data was collected. The animal body temperature was monitored continuously using a rectal probe. Blood pressure, heart beat, ETCO2 were also monitored continuously. Monkeys were scanned under 1-1.5% isoflurane anesthesia. Data processing including motion correction, image registration, apparent diffusion coefficient (ADC) map calculation from the serial diffusion MRI data for quantitative analyst was conducted by using the FSL software and Matlab scripts. The ADC values in the cerebrospinal fluid (CSF) ventricle were used to examine the progressive changes of the monkey’s brain temperature during prolonged isoflurane administration [2, 3]. The dMRI-derived temperature values were normalized to the averaged core temperatures of each animal for comparison purpose. All animals were recovered successfully after each MRI session. All the procedures were approved by IACUC of the institution.Results
The phantom tests illustrated that the dMRI measures with b=1000 s/mm2 showed better accuracy with the temperature changes of 1 and 2.5°C than those with b=400 s/mm2 (correlation coefficient R= 0.987 vs. 0.958) (Fig 1). Accordingly, the dMRI measures with b=1000 s/mm2 were evaluated in the following animal scans. The progressive changes of the brain and core temperatures of each monkey were illustrated in Figure 2. For the 4 monkeys, the core temperatures varied between 35.8 and 36.7°C during the entire scanning session. The temporal changes of dMRI-derived brain temperatures were in good agreement with the core value after ~90 minute isoflurane administration. However, the dMRI-derived brain temperatures were obviously higher than the core temperatures between 50-80 minutes of isoflurane administration in each monkey.Discussion
The rectal temperature is generally used to examine the effects of PIH on the brain temperatures of the experimental animals in stroke studies [4, 5]. However, the body and brain temperature differences have been seen in TBI or patients with other cerebral injuries previously [6-8]. General anesthesia is normally applied during neuroimaging study of animal models and could induce hypothermia as well[9]. Anesthetics like isoflurane can affect the brain metabolites and physiology like cerebral blood flow (CBF) which could cause temperature fluctuations in experimental animals [10-12]. Therefore, the accurate estimate of the brain temperature is critical for properly evaluating the neuroprotective effects of PIH in experimental models (especially in large animals like primates or pigs).The results on phantom tests demonstrated the diffusion MRI could be a robust approach to monitor the mild temperature changes (1-2.5°C) of water. The preliminary results in monkeys showed its dependence of accuracy on the duration of isoflurane administration, suggesting the anesthesia administration has evident effects on the brain temperature during the initial period of induction, in agreement with the previous rodent study [13]. Interestingly, the dMRI-derived temperatures were overestimated for the temperature measures during early isoflurane administration, the further investigation is warranted.
The body temperature varies from subject to subject as usually seen in human and rhesus monkeys in awake and anesthetized condition. As reported in the present study, the pattern of temporal changes in the core temperature of each monkey were different from each other. Therefore, the results of each monkey was exhibited independently in order to demonstrate inter-subject difference and also the robustness of the dMRI measurement in each subject.
Conclusion
Therapeutic hypothermia represents one of the most potent neuroprotectants in stroke and traumatic brain injury study, and large animal models are increasingly used because their translational potential. The preliminary results suggest the dMRI thermometry may be a non-invasive and robust approach to examine the effects of the pharmacologically-induced hypothermia on the brain temperature changes in large animal models but must be used with caution as the dMRI-derived temperatures may be biased due to anesthesia administration during the initial period. Also, the temperature difference between the brain and core should be considered in preclinical and clinic studies of therapeutic hypothermia[14].Acknowledgements
No acknowledgement found.References
[1] A.M. Kuczynski, S. Marzoughi, A.S. Al Sultan, F. Colbourne, B.K. Menon, A. van Es, A.L. Berez, M. Goyal, A.M. Demchuk, M.A. Almekhlafi, Therapeutic Hypothermia in Acute Ischemic Stroke-a Systematic Review and Meta-Analysis, Curr Neurol Neurosci Rep, 20 (2020) 13.
[2] L.R. Kozak, M. Bango, M. Szabo, G. Rudas, Z. Vidnyanszky, Z. Nagy, Using diffusion MRI for measuring the temperature of cerebrospinal fluid within the lateral ventricles, Acta Paediatr, 99 (2010) 237-243.
[3] K.M. Hasan, J.A. Lincoln, F.M. Nelson, J.S. Wolinsky, P.A. Narayana, Lateral ventricular cerebrospinal fluid diffusivity as a potential neuroimaging marker of brain temperature in multiple sclerosis: a hypothesis and implications, Magn Reson Imaging, 33 (2015) 262-269.
[4] S. Wang, X. Gu, R. Paudyal, L. Wei, T.A. Dix, S.P. Yu, X. Zhang, Longitudinal MRI evaluation of neuroprotective effects of pharmacologically induced hypothermia in experimental ischemic stroke, Magn Reson Imaging, 40 (2017) 24-30.
[5] S. Wei, J. Sun, J. Li, L. Wang, C.L. Hall, T.A. Dix, O. Mohamad, L. Wei, S.P. Yu, Acute and delayed protective effects of pharmacologically induced hypothermia in an intracerebral hemorrhage stroke model of mice, Neuroscience, 252 (2013) 489-500.
[6] C. Childs, K.W. Lunn, Clinical review: Brain-body temperature differences in adults with severe traumatic brain injury, Crit Care, 17 (2013) 222.
[7] K.W. Lunn, C. Childs, A systematic review of differences between brain temperature and core body temperature in adult patients with severe traumatic brain injury, JBI Libr Syst Rev, 10 (2012) 1410-1451.
[8] S. Rossi, E.R. Zanier, I. Mauri, A. Columbo, N. Stocchetti, Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage, J Neurol Neurosurg Psychiatry, 71 (2001) 448-454.
[9] B. Bindu, A. Bindra, G. Rath, Temperature management under general anesthesia: Compulsion or option, J Anaesthesiol Clin Pharmacol, 33 (2017) 306-316.
[10] C.X. Li, X. Zhang, Effects of Long-Duration Administration of 1% Isoflurane on Resting Cerebral Blood Flow and Default Mode Network in Macaque Monkeys, Brain Connect, 7 (2017) 98-105.
[11] C.X. Li, X. Zhang, Evaluation of prolonged administration of isoflurane on cerebral blood flow and default mode network in macaque monkeys anesthetized with different maintenance doses, Neurosci Lett, 662 (2018) 402-408.
[12] Z. Liang, X. Liu, N. Zhang, Dynamic resting state functional connectivity in awake and anesthetized rodents, Neuroimage, 104 (2015) 89-99.
[13] M.J. Shirey, J.B. Smith, D.E. Kudlik, B.X. Huo, S.E. Greene, P.J. Drew, Brief anesthesia, but not voluntary locomotion, significantly alters cortical temperature, J Neurophysiol, 114 (2015) 309-322.
[14] M.A. Landry, L.W. Doyle, K. Lee, S.E. Jacobs, Axillary temperature measurement during hypothermia treatment for neonatal hypoxic-ischaemic encephalopathy, Arch Dis Child Fetal Neonatal Ed, 98 (2013) F54-58.Figures
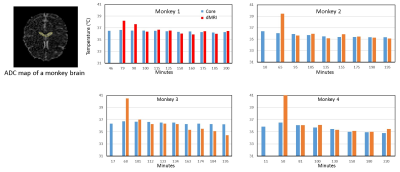
Fig 2. Demonstration of progressive temperature changes in the monkey brains (n=4) maintained under isoflurane anesthesia.
The results of diffusion MRI thermometry (b-value = 1000 s/mm2) were compared with the core temperatures for each monkey.
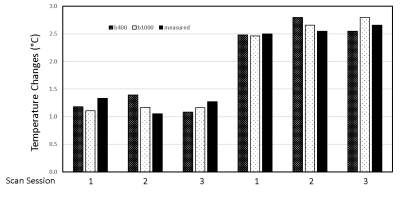
Fig 1. Demonstration of water phantom results measured using diffusion MRI thermometry (b-value = 400, 1000 s/mm2)
with the temperature changes of ~1 and 2.5 °C.