2314
Non-Invasive Multi-Modal Imaging of Body Composition and Bone Density in Genetically Modified Fshb Mice
Natalie Serkova1, Julia E Slack2, Jenna L Steiner3, Anna De Schutter3, Aaya AlKassi3, Jamie Henry4, and Mark S Brown3
1Radiology, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 2Emory University, Atlanta, GA, United States, 3University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 4Colorado State University, Fort Collins, CO, United States
1Radiology, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 2Emory University, Atlanta, GA, United States, 3University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 4Colorado State University, Fort Collins, CO, United States
Synopsis
We report on a multi-modal quantitative MRI/ CT imaging protocol for characterizing the comprehensive body composition and bone health in a genetically modified mouse model. Follicle stimulating hormone (FSH) is directly involved in the regulation of estrogen production, osteoclasto-genesis, and adiposity. In this study we show that the deletion of FSH gene (-/-) in the mouse results in increased adiposity and decreased tibia density in male and female mice as they age. Multi-parametric imaging provides a unique opportunity to quantify visceral adiposity levels, muscle mass, and bone density in mutant animals, non-invasively and in real time.
Introduction
The assessment of body composition, especially in the relation to obesity, has relied on a few anthropometric measurements, including BMI, waist circumference of waist-to-hip ratio. However, these indirect measurements are unable to distinguish fat from skeletal muscle, nor can they distinguish between visceral and subcutaneous fat. Gonadotropins are pituitary gonadotrope-derived glycol-protein hormones, including luteinizing hormone (LH) and follicle stimulating hormone (FSH). FSH plays important roles in aging, fertility, as well as pathogenesis and progression of epithelial ovarian cancer (Song et al., 2020). It has been shown that FSH is directly involved in the regulation of estrogen production, osteoclasto-genesis, and adiposity (Gilbert al., 2018). The goal of this study was to assess the sex- and age-dependent differences in body composition and bone density in wild-type (+/+) and genetically modified Fshb mutant (-/-) mice using non-invasive high-resolution imaging methods (MRI and CT).Methods
All animal protocols were reviewed and approved by the University of Colorado IACUC. Female and male mice were included in both wild-type (+/+) and Fshb -/- mutant phenotype analyses, at young (3-4 months) and old (8 months) age (8 groups, total 46 animals). Mice were induced using anesthesia (2% isoflurane). First, proton-density RARE fat-suppressed and non-suppressed MRI scans were acquired on a Bruker 9.4 Tesla BioSpec MRI using a mouse bird-cage body coil. The respiratory motion was monitored with an MRI compatible gating system (SA Instruments). Then, CT scans were acquired using a Siemens Inveon CT using the whole body FOV. A medium resolution/ low dose CT scan were acquired with the Xray source set to 160 mA current and 90kVp voltage. Analytical methodologies included (i) volumetric analysis of muscles and total body fat using Bruker ParaVision NEO360 software; and (ii) bone density of the tibia, femur, and lumbar spine of each mouse using Siemens Inveon IRW and density-phantom generate curves to report Hounsfield Units (HU). In-house MATLAB simulation and a new algorithm was developed for automatic image segmentation and quantification of visceral and subcutaneous fat volumes. Total fat and muscle volume was achieved by summing the individual fat volume across all anatomical slices. The image analysis researcher was blinded to age and genotypes of mice. ANOVA was performed to determine statistical significance and correlation analysis.Results
Muscle volume, fat volume and distribution in the abdominal and gluteal regions were assessed by proton-density (PD) weighted MRI. Contiguous 1-mm thick, no gap transversal and coronal slices throughout the regions were analyzed based on the auto-thresholding function of the MATLAB package (Figure 1), using fat-suppressed axial images for total muscle volume and non-suppressed coronal images for total fat deposition (Figure 1). Deletion of FSH gene (-/-) results in increased adiposity and decreased tibia density in male and female mice as they age - Indeed, the 3D-CT reconstruction clearly shows a declined bone density as a function of increased visceral and subcutaneous fat deposition by MRI in -/- animals (Figure 2). In general, there was a strong correlation between body fat and femur/tibia bone density (r=-0.85,-0.83 respectively, Figure 3). Older -/- knockout mice had lower femur and tibia bone density than younger knockout mice (p<.05, Figure 4). Finally, the males showed significant higher muscle mass and tibia bone density compared to the females.Discussion
Deletion of FSH gene (-/-) results in increased adiposity and decreased tibia density in male and female mice as they age. In old Fshb -/- female, body weight, although slightly elevated, was not statistically significant from young wild-type animals - whereas their fat deposition was significantly elevated by MRI, indicating the need of imaging assessment. MRI and CT acquisitions on the same animal provide complementary information on metabolic changes in body composition of genetically modified animals.Acknowledgements
The study was supported by the NIH Shared Instrumentation Grant Program (S10 OD023485 and S10 OD027023), the University of Colorado Cancer Center grant (P30 CA046934), the NIH 1R25CA240122-01, and the Cancer League of Colorado.References
No reference found.Figures
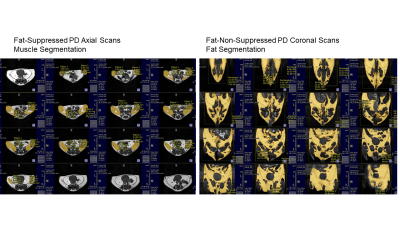
Figure 1. Volumetric muscle quantification using
Paravision NEO360 on the axial plane and fat quantification on the coronal
plane
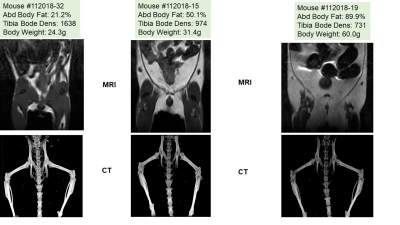
Figure 2. MRI images of mice illustrating various
levels of adiposity storage by MRI and bone density by 3D-CT
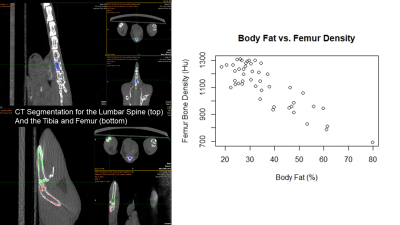
Figure 3. Lumbar spine, femur and tibia
segmentation for bone density analysis on CT, as well as the correlation
analysis between body fat and bone density
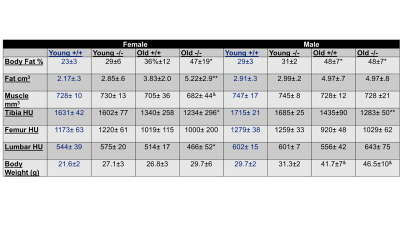
Figure
4. The summary table on body composition (fat and muscle volumes) and bone
density in wild type and Fshb mutant (-/-) mice with observed sex- and
age-differences
DOI: https://doi.org/10.58530/2022/2314