2312
Peri-conceptional Alcohol Exposure Shapes Placental Functions in Fetal Alcohol Spectrum Disorder1Developmental Biology, University of Pittsburgh, Pittsburgh, PA, United States, 2Obstetrics, Gynecology & Reproductive Sciences, University of Pittsburgh, Pittsburgh, PA, United States, 3Pediatrics, University of Pittsburgh, Pittsburgh, PA, United States, 4Biomedical Engineering, University of Pittsburgh, Pittsburgh, PA, United States, 5Physiology, University of Cambridge, Cambridge, United Kingdom, 6Biological Regulation, Weizmann Institute, Rehovot, Israel, 7Cedars Sinai Medical Center, Los Angeles, CA, United States
Synopsis
Peri-conceptional alcohol (PCA) exposure leading to fetal alcohol spectrum disorder continues to a significant public health concern because alcohol use ceases only after recognition of pregnancy weeks after conception, but the teratogenic damages have already occurred. Our study showed that PCA exposure in mice resulted in compromised placental perfusion and placental capability to adapt to acute hypoxia challenges. Placental abnormalities due to PCA may further exacerbate fetal neurodevelopmental deficits.
INTRODUCTION
Despite stern public health warnings that no point in pregnancy is safe for alcohol consumption, fetal alcohol spectrum disorder (FASD)[1, 2] continues to be a significant public health concern. Pertinent to this issue is the difficulty in reducing women’s alcohol consumption around conception. Alcohol use ceases only after recognition of pregnancy, several days to weeks after conception. Unfortunately, any teratogenic damage to oocytes, zygotes and pre-implantation blastocysts of peri-conceptional alcohol (PCA) exposure have already occurred before the awareness of pregnancy.The placenta is vital for fetal development, as is responsible for materno-fetal nutrient and oxygen transfer exchange. Placenta-related complications[3, 4] are associated with alcohol use during pregnancy, including placental insufficiency, miscarriage, pre-term birth, still birth, and intrauterine growth restriction. Placental health greatly impacts the fetal neurodevelopment, recognized as the “placenta-brain axis”[5-8]. We have established a motion-and-time resolved 4D functional MRI (4D-fMRI)[9, 10] capable of 3D isotropic MRI time-series to simultaneously capture anatomical and BOLD signals in the same scan with both high-spatial and high-temporal resolution (frame rate: ~14 msec, voxel size: 0.00047 mm3) using sub-Nyquist sparse sampling. We further developed it into 4D oxy-wavelet MRI (4D-fMRI in conjunction with oscillating hypoxia challenges) to probe placental and fetal brain’s capability to adapt to acute hypoxia challenges. The goal of this study is to investigate the effect of PCA exposure in mice on the developing placenta and fetal brain.
METHODS
Animal model: PCA[11] in C57BL6/J mice was initiated by ad libitum intake of 12.5% alcohol for the 4 days immediately preceding and 4 days after conception before implantation. The pregnant female mice were subjected to in vivo MRI on embryonic day E9.5, E14.5 and E17.5. In others, mice were allowed to litter and pup viability assessed at birth.In vivo MRI: In vivo MRI for the pregnant females were acquired at a preclinical 7-Tesla MRI (Bruker BioSpec USR 70/30) with a 35-mm quadrature volume coil, with 1.5% isoflurane anesthesia.
Placental perfusion with dynamic contrast enhancement (DCE): In vivo placental perfusion was quantified by DCE MRI with a single bolus gadolinium (Gd) contrast agent (MultiHance, 0.1 mmol/kg bodyweight) injection using the steepest slope model[12, 13].
Placental compensation to cyclic hypoxia with 4D oxy-wavelet MRI: We express a dynamic blood oxygenation level dependent (BOLD) signal (for spatial position and time ) as the product of basis images and temporal functions. It exploits correlation of images over time[14]; further modeling the as transform sparse[15] during image reconstruction allows 4D acquisition with both high spatial and high temporal resolutions in the same single scan. This allows for fMRI with high spatiotemporal resolutions without common drawbacks of conventional fMRI such as spatial-temporal resolution tradeoff and co-registration errors. Furthermore, it can assess oxygen attenuation during the same single scan, whereas conventional fMRI needs to acquire each oxygen condition sequentially. 4D-fMRI was acquired with FOV=4.5cm×3cm×2cm, isotropic voxel size 120μm×120μm×120μm, FA=10°,TE=4.5ms, TR=8.3ms, total scan time=40min. During acquisition, short bursts of 3-min hypoxia (10% O2) interleaved with 3-min hyperoxia (100% O2) were supplied via a nose cone to the pregnant females.
Ex vivo high-resolution MRI: Following in vivo MRI, embryos and placentas were fixed for high-resolution ex vivo MRI at 7-Tesla with a fast spin echo sequence with the following parameters: FOV= 40×14×9 mm3, matrix size=1024×304×196, voxel size=39×46×46μm3, TE=12.3ms, RARE factor=8, effective TE=24.7ms, TR=900ms.
RESULTS
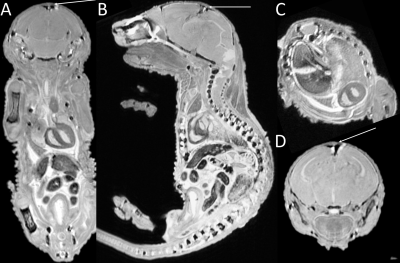
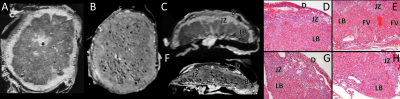
Placental abnormalities including impaired blood flow are seen in FASD placentas. We quantified placental perfusion by dynamic contrast enhanced (DCE) MRI (Fig.1). We observed different placental perfusion pattern in PCA (Fig.1C) compared to normal (Fig.1B) with quantitative DCE. Placental perfusion was quantified with the steepest slope model[12, 13] (Fig.1D). PCA placenta showed reduced placental perfusion (Fig.1F). Efficient oxygen delivery to placenta and fetus are essential for fetal brain development, and feto-placental hypoxia and impaired response to hypoxia are associated with FASD. Placenta and fetal brain responses to acute hypoxia challenges were measured with 4D oxy-wavelet MRI (Fig.2). When challenged with oscillating short bursts of 3-min hypoxia (10% O2) via maternal inhalation (Fig.2AB, dark blue periods), control fetal brains and placentas (Fig.2 A,C) can adapt to the hypoxia challenges and maintain a high oxygenation levels (Fig.2EF purple). On the contrary, PCA fetal brains and placentas (Fig.2 B,D) were unable to adapt to hypoxia challenges, and remained in negative (lower than baseline) oxygenation states (Fig.2EF green). PCA fetal brains and placentas showed poor responses to hypoxia challenges, indicating PCA exposure affecting both fetal brains and placental development. With this PCA voluntary drinking protocol, ~37.8 % of pups were born dead. Ex vivo MRI of neonates on postnatal day P0 showed subarachnoid hemorrhage (Fig.3) in the stillborn pups. Placentas harvested on embryonic day E17.5 from alcohol drinking females displayed abnormal expansion of trophoblast giant cell layer and disorganized junctional zone (Fig.4B, F-G). The labyrinth layers, important for mediating maternal-fetal blood exchange, were disorganized with reduced vascularity. In control placenta fetal vessels (FV) orderly projected through the labyrinth (LB), but this was not seen in alcohol affected placenta (Fig.4C-H).CONCLUSION
Our study showed that PCA exposure in mice mice resulted in structural and functional placental abnormalities, which may exacerbate neurodevelopmental deficits.Acknowledgements
MSC and YLW are supported by funding from NIH-R21-EB023507, AHA-18CDA34140024, and DoD-W81XWH1810070.References
1. Wozniak, J.R., E.P. Riley, and M.E. Charness, Clinical presentation, diagnosis, and management of fetal alcohol spectrum disorder. Lancet Neurol, 2019. 18(8): p. 760-770.
2. Hoyme, H.E., et al., A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics, 2005. 115(1): p. 39-47. 3. Gundogan, F., et al., Dose effect of gestational ethanol exposure on placentation and fetal growth. Placenta, 2015. 36(5): p. 523-30.
4. Burton, G.J., A.L. Fowden, and K.L. Thornburg, Placental Origins of Chronic Disease. Physiol Rev, 2016. 96(4): p. 1509-65.
5. Mao, J., et al., Bisphenol A and bisphenol S disruptions of the mouse placenta and potential effects on the placenta-brain axis. Proc Natl Acad Sci U S A, 2020. 117(9): p. 4642-4652.
6. Rosenfeld, C.S., The placenta-brain-axis. J Neurosci Res, 2020.
7. Behura, S.K., et al., The brain-placental axis: Therapeutic and pharmacological relevancy to pregnancy. Pharmacol Res, 2019. 149: p. 104468.
8. Hsiao, E.Y. and P.H. Patterson, Placental regulation of maternal-fetal interactions and brain development. Dev Neurobiol, 2012. 72(10): p. 1317-26.
9. Christodoulou, A.G., et al. Fetal Brain-Heart-Placental Interactions with Acute Hypoxia Challenge in Genetic Mouse Models of Hypoplastic Left Heart Syndrome with in utero 4D Dynamic MRI. in ISMRM International Society of Magnetic Resonance in Medicine. 2019. Montreal, QC, Canada.
10. Christodoulou, A.G., et al., 4D Real-time BOLD MRI in Genetically Engineered Mouse Brains with Acute Hypoxia Challenge, in International Soceity of Magnetic Resonance in Medicine (ISMRM). 2019: Montreal, QC, Canada, .
11. Kalisch-Smith, J.I., et al., Periconceptional alcohol exposure causes female-specific perturbations to trophoblast differentiation and placental formation in the rat. Development, 2019. 146(11).
12. Plaks, V., et al., Functional Phenotyping of the Maternal Albumin Turnover in the Mouse Placenta by Dynamic Contrast-Enhanced MRI. Molecular Imaging and Biology, 2011. 13(3): p. 481-492.
13. Bao, Q., et al., Diffusion and perfusion MRI of normal, preeclamptic and growth-restricted mice models reveal clear fetoplacental differences. Sci Rep, 2020. 10(1): p. 16380.
14. Liang, Z.-P., Spatiotemporal imaging with partially separable functions. Proc IEEE Int Symp Biomed Imaging, 2007: p. 988-991.
15. Lustig, M., D. Donoho, and J.M. Pauly, Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med, 2007. 58(6): p. 1182-95.
Figures