2308
Optimized U-net++: Novel Algorithm for Sub-regional Segmentation of Knee Cartilage and Bone1Department of Medical Imaging, The Third Affiliated Hospital of Southern Medical University, Guangzhou, China, 2School of Electronics and Communication Engineering, Sun Yat-sen University, Shenzhen, China, 3China International Center, Philips Healthcare, Guangzhou, China
Synopsis
Cartilage degeneration and subchondral bone alterations play an important role in the pathogenesis and progression of knee osteoarthritis (OA). MRI can detect morphological or compositional change of cartilage and bone. Regional analysis of cartilage and bone lesions would significantly improve the diagnosis of OA and help to understand its role in OA. Automated segmentation of cartilage and bone on MRI is a necessary first step for quantitative measures. Therefore, we proposed an optimized U-net (PSA-U-net++) to solve the problem of sub-regional segmentation of bone and cartilage. The initiatory results showed that our model can accurately segment cartilage and bone.
Purpose and Introduction
Knee osteoarthritis (OA) is a chronic, degenerative joint disease that affects many parts of the whole joint, including articular cartilage, subchondral bone, synovium, ligaments and periarticular muscles. Cartilage degeneration is often the early hallmark of osteoarthritis (OA), which is characterized by significant changes in structure and composition[1]. In addition, some studies suggested that early degeneration in cartilage also impaired subchondral bone and caused bone remodeling and bone marrow lesions[2]. MRI can assess cartilage and bone health. Based compositional quantitative data (such as the value of T1rho, T2 value, T2* and MMF of cartilage) and morphological quantitative data (such as articular cartilage volume and thickness or BMLs size) have become important imaging biomarkers for studying the early diagnosis and prognosis of OA[3-5]. However, these quantitative image-based biomarkers require precise segmentations. At present, a large number of studies have been carried out to explore the methods to automatic segmentation of cartilage and bone, and some progress has been made[4, 6]. Nevertheless, there is little literature on sub-regional automatic segmentation of cartilage and bone. Sub-regional automatic segmentation of cartilage and bone is helpful for spatial pattern analysis, which will further improve diagnosis and comprehensive understanding of pathogenesis of OA. Therefore, we developed a new deep learning-based model to sub-regional segmentation of knee bone and cartilage on MRI images from Osteoarthritis Initiate (OAI).Materials and Methods
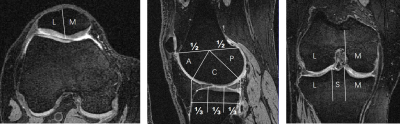
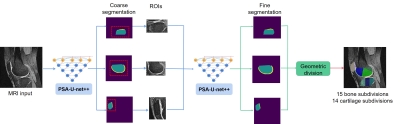
In this study, 100 cases were randomly selected from the OAI-ZIB dataset. 3D dual echo steady state (DESS) images were used. According to MOAKS, cartilage and bone are divided into 15 and 14 subregions, respectively[7]. The demographics and clinical characteristics of the participants can be showed in Table 1 and the delineation of knee cartilage and bone subregional divisions are demonstrated in Figure.1. We designed an optimized U-net++[8] (polarization self-attention U-net++, PSA-U-net++) for automatic segmentation of knee cartilage and bone subdivisions. The proposed segmentation framework is illustrated in Figure 2. Studies were split into an internal training set (70%), validation set (15%), and test set (15%). The Dice Similarity Coefficient (DSC) was calculated to evaluate the accuracy of our method. In addition, the proposed PSA-U-net++ CNN model was compared with U-net++ and U-net.Results and discussion
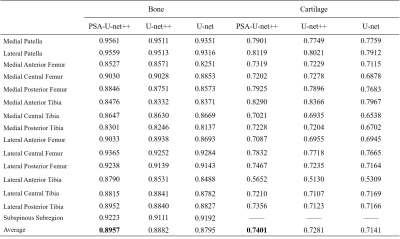
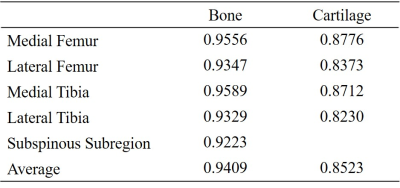
The automatic segmentation times of knee bone and cartilage subdivisions based on MOAKS were on average 28s (Runs on a RTX 2080 Super card). Summary statistics of segmentation accuracies are displayed in Table.2. The mean dice similarity coefficient (DSC) of 15 bone subdivisions was 0.8957, ranging from 0.8301 in lateral posterior femur to 0.9561 in medial patella. For automatic segmentation of cartilage 14 partition, the mean DSC is 0.7401, of which, the anterior lateral femur achieves 0.8290. Comparing the U-net++ and U-net model to our optimized model, the PSA-U-net++ model outperformed in the bone and cartilage segmentation, which can be viewed in Table 2. Moreover, when the articular surface of femur and tibia is divided according to the medial and lateral compartments, the segmentation accuracies were further improved. The detailed results were presented in Table 3. Although our model shows great performance, there are several limitations. The major limitation is that the model only trained on DESS MRI images. Therefore, the further step of our research is transferring the model to more MRI sequences to improve its generalization.Conclusion
The PSA-U-net++ CNN model shows promising segmentation performance for knee bone and cartilage subdivisions from MRI data, which will be expected to be an auxiliary tool for the comprehensive assessment of articular cartilage and bone.Acknowledgements
Lijie Zhong and Shaolong Chen contributed equally to this work.
Xiaodong Zhang and Zhiyong Zhang are both corresponding authors.
References
[1] Eckstein F, Wirth W, Culvenor AG. Osteoarthritis year in review 2020: imaging[J].Osteoarthritis Cartilage, 2021, 29(2): 170-179.
[2] Lories RJ, Luyten FP. The bone–cartilage unit in osteoarthritis[J].Nature Reviews Rheumatology, 2010, 7(1): 43-49.
[3] Chalian M, Li X, Guermazi A ,et al. The QIBA Profile for MRI-based Compositional Imaging of Knee Cartilage[J].Radiology, 2021, 301(2): 423-432.
[4] Xue YP, Jang H, Byra M ,et al. Automated cartilage segmentation and quantification using 3D ultrashort echo time (UTE) cones MR imaging with deep convolutional neural networks[J].Eur. Radiol., 2021, 31(10): 7653-7663.
[5] Li X, Ma BC, Bolbos RI ,et al. Quantitative assessment of bone marrow edema-like lesion and overlying cartilage in knees with osteoarthritis and anterior cruciate ligament tear using MR imaging and spectroscopic imaging at 3 Tesla[J].J. Magn. Reson. Imaging, 2008, 28(2): 453-461.
[6] Ambellan F, Tack A, Ehlke M ,et al. Automated segmentation of knee bone and cartilage combining statistical shape knowledge and convolutional neural networks: Data from the Osteoarthritis Initiative[J].Med. Image Anal., 2019, 52: 109-118.
[7] Hunter DJ, Guermazi A, Lo GH ,et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score)[J].Osteoarthritis Cartilage, 2011, 19(8): 990-1002.
[8] Zhou ZW, Siddiquee MMR, Tajbakhsh N, et al. UNet++: Redesigning skip connections to exploit multiscale features in image segmentation [J]. IEEE Transactions on Medical Imaging, 2020, 39(6): 1856-1867.
Figures
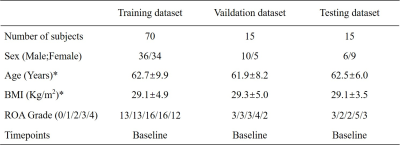
Table 1. Patient Demographic and Clinical Characteristics
Note—* data are means ± standard deviations, other data are numbers of patients.