2288
Variable density Poisson disc acquisition with iterative deep learning reconstruction for highly accelerated 3D T1-weighted abdominal imaging1GE Healthcare, Madison, WI, United States, 2GE Research, Niskayuna, NY, United States, 3GE Research, Herzliya, Israel, 4GE Healthcare, Waukesha, WI, United States, 5GE Healthcare, Rochester, MN, United States, 6GE Healthcare, Menlo Park, CA, United States, 7GE Healthcare, Houston, TX, United States
Synopsis
3D T1-weighted gradient echo imaging is a key component of the MRI assessment of the abdomen, particularly for the identification and characterization of liver tumors, however, significant acceleration is necessary to consistently mitigate respiratory motion artifact. A variable density Poisson disc undersampled acquisition with a densely connected iterative deep convolutional neural network reconstruction was developed to provide next-generation acceleration up to a factor of 10. On retrospectively undersampled data, the technique outperformed compressed sensing reconstruction in terms of normalized mean-squared error and structural similarity; with a prospectively undersampled scan, the technique maintained image quality in terms of artifact and contrast.
Introduction
3D T1-weighted gradient echo imaging is a key component of the MRI assessment of the abdomen, particularly for the identification and characterization of liver tumors. Although 3D acquisition provides high SNR efficiency and volumetric coverage, the scan is sensitive to respiratory motion, so the use of breath-holding, gating, or intrinsically motion-robust acquisition, for example, is necessary. However, these motion artifact mitigation techniques, in turn, limit the duration or temporal resolution of a scan that is usually performed dynamically with contrast, so properly timing contrast arrival and distinguishing arterial, portal venous, and delayed phases become challenging. Acceptable image quality is possible with state-of-the-art acceleration via parallel imaging and compressed sensing along with optimized coil geometry. Still, in order to 1) consistently address patients with poor breath-holding capability and/or large habitus, 2) improve temporal resolution, 3) expand coverage to the pelvis, or 4) attain isotropic resolution for reformatting, next-generation acceleration would be required. In this work, a highly undersampled acquisition is paired with a deep learning-based reconstruction to yield up to 10× acceleration.Methods
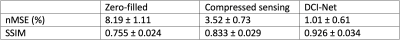
For deep learning-based image reconstruction from undersampled k-space data, we used DCI-Net (Densely Connected Iterative Network)1 with 28 iterations, 9 convolutional layers per iteration, 96 2D convolution kernels (applied to phase-slice encoding planes) per layer, and up to 20 skip connections per iteration. The DCI-Net was trained using 149 3D T1-weighted brain data sets. For the loss function, we used a contrast-weighted structural similarity (SSIM)2 extended to complex-valued images where the weights for the luminance, contrast, and structure comparison functions were 0.3, 1, and 0.3, respectively. In this study, we focus on variable density Poisson disc (VDPD) undersampling.3To quantitatively evaluate how well the DCI-Net can reconstruct images, we first used retrospectively undersampled k-space data, for which ground-truth fully-sampled data are known. For the evaluation, we used 15 diverse mildly-undersampled 3D T1-weighted abdomen data sets with 2× or less acceleration. For each data set, we calculated ground-truth fully-sampled k-space data using Autocalibrating Reconstruction for Cartesian imaging (ARC) and then retrospectively undersampled with up to 10× acceleration (mean net acceleration 9.4 with standard deviation 0.3) using a VDPD sampling pattern. We compared DCI-Net reconstructed images to fully-sampled images using normalized mean-squared error (nMSE) and SSIM. For comparison, we also included images reconstructed using a total-variation-based compressed sensing method4 that employed data-driven iterative soft thresholding.
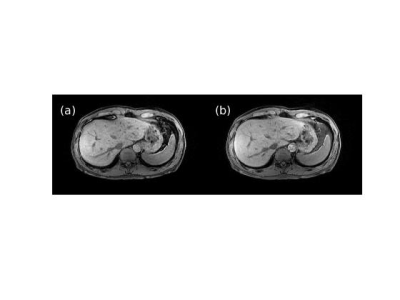
Next, we evaluated the technique with a prospectively undersampled acquisition in healthy adult volunteers without contrast. IRB approval was granted to scan up to 50 subjects on a 3-T whole-body system (Architect, GE Healthcare, Waukesha, WI, USA). Breath-hold axial 3D T1-weighted LAVA (Liver Acquisition with Volume Acceleration) scans of the upper abdomen were performed with the following base protocol: flip angle: 12°, TE/TR: 1.9/4.1 ms, intermittent adiabatic fat saturation preparation time: 24 ms, receive bandwidth: 62.5 kHz, FOV: 42.0 × 37.8 cm, slice thickness: 4.0 cm, frequency (right-left)/phase (anterior-posterior) encodings: 300 × 200, acquired slices: 48, slice partial Fourier factor: 0.71, ARC acceleration factor: 2 × 1.25, scan time: 11.8 s. Scans were then repeated with a VDPD undersampling factor that resulted in a matching scan time as well as larger factors up to 10 to achieve a scan time as short as 4.9 s. Finally, the scanning procedure was duplicated except with a pencil beam navigator tracker placed across the diaphragm to prospectively gate the acquisition during the exhalation phase of free breathing. Estimated scan times for the ARC and VDPD navigator protocols were 41.7 and 16.4 s, respectively. Images were subjectively assessed for acceleration-related artifact and changes in contrast.
Results
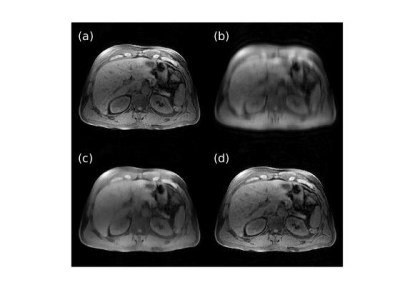
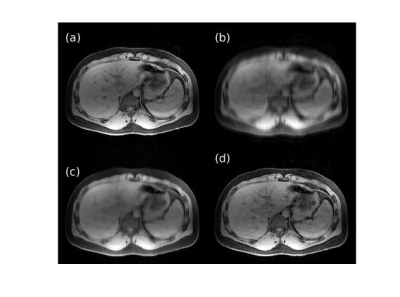
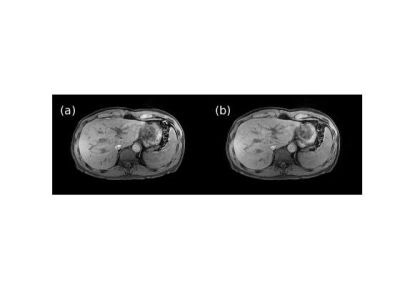
Figures 1 and 2 show representative images reconstructed from retrospectively VDPD undersampled data. Table 1 gives a comparison of the image metrics (nMSE and SSIM) relative to ground-truth fully-sampled images.Figures 3 and 4 show representative images reconstructed from prospectively undersampled data for breath-hold and navigator scans, respectively.
Discussion
For retrospectively undersampled data sets, DCI-Net outperformed total-variation compressed sensing reconstruction in terms of nMSE and SSIM. Acceptable image quality was achieved at 10× acceleration in comparison to ground-truth images. For prospectively undersampled data sets, acceleration-related artifact and contrast changes were clinically insignificant even at 10× acceleration.The DCI-Net was purposefully trained using brain data sets, which is worthwhile to note because abdomen data tends to be of lower quality in terms of motion artifact, B0/B1 inhomogeneity, resolution, and overall SNR. While this study focused on using additional acceleration to maintain image quality at a lower scan time, certainly there is flexibility to apply the acceleration appropriately to better answer a specific clinical question, which may mean larger coverage or improved resolution, for example.
Conclusion
Variable density Poisson disc undersampled acquisition with DCI-Net deep learning-based reconstruction achieves 10× acceleration for 3D T1-weighted gradient echo imaging of the abdomen, where significant acceleration is necessary to consistently mitigate respiratory motion artifact.Acknowledgements
No acknowledgement found.References
- Malkiel I, Ahn S, Slavens Z, et al. Densely connected iterative network for sparse MRI reconstruction. ISMRM 2018, p. 3363.
- Ahn S, Menini A, McKinnon G, et al. Contrast-weighted SSIM loss function for deep learning-based undersampled MRI reconstruction. ISMRM 2020.
- Moghari MH, Uecker M, Roujol S, et al. Accelerated whole-heart MR angiography using a variable-density poisson-disc undersampling pattern and compressed sensing reconstruction. Magn Reson Med. 2018;79(2):761-769.
- Khare K, Hardy CJ, King KF, et al. Accelerated MR imaging using compressive sensing with no free parameters. Magn Reson Med 2012;68(5):1450-1457.
Figures