2284
Optimal Transport driven Cycle-consistent Generative Adversarial Network (OT-CycleGAN) for an accurate MR water-fat separation1Biomedical Imaging Center, Pontificia Universidad Católica de Chile, Santiago, Chile, 2ANID – Millennium Science Initiative Program – Millennium Nucleus for Cardiovascular Magnetic Resonance, Santiago, Chile, 3Electrical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile, 4Institute for Biological and Medical Engineering, Pontificia Universidad Catolica de Chile, Santiago, Chile, 5Radiology Department, School of Medicine, Pontificia Universidad Catolica de Chile, Santiago, Chile, 6Institute for Mathematical & Computational Engineering, Pontificia Universidad Catolica de Chile, Santiago, Chile, 7ANID – Millennium Science Initiative Program – Millennium Nucleus for Discovery of Structure in Complex Data, Santiago, Chile
Synopsis
MRI water-fat separation is an ill-posed inverse problem usually addressed using complex numerical methods. This problem is related to an MR signal physical model that includes the effects of R2* signal decay ratio and main magnetic field inhomogeneities (Δf), which induce non-linearities that are related to the ill-posedness of the inverse problem. In this work, we propose an Optimal Transport driven Cycle-consistent Generative Adversarial Networks (OT-CycleGAN) framework, which is physics-based, and could use partially labeled training data to estimate the non-linear components (R2* and Δf), and posteriorly compute the water-fat images through a conventional least-squares approach.
Introduction
Non-Alcoholic Fatty Liver Disease (NAFLD) is a common condition worldwide, with an estimated global prevalence of 25%[1]. Liver fat fraction is the most relevant biomarker to diagnose this disease, and Proton Density Fat Fraction ($$$PDFF$$$$) is a highly correlated metric that could be measured non-invasively by solving the MR water-fat separation problem. However, to accurately solve it, a complex MR signal model should be considered, which include the multi-peak spectrum of fat[2], and the non-linear effects of $$$R_2^*$$$ signal decay and main magnetic field inhomogeneities ($$$\Delta f$$$). The current gold standard to solve this problem are iterative algorithms[3,4], although they show artifacts in some cases. Deep Learning (DL) methods have become a promising alternative due to their accuracy and artifact correction [5–7].In this work, we propose an unsupervised DL approach based on an Optimal Transport driven Cycle-consistent Generative Adversarial Network (OT-CycleGAN)[8] to solve the non-linear component of the signal ($$$R_2^* , \Delta f$$$), and subsequently separate the water and fat signals through a linear least-squares estimation.
Methods
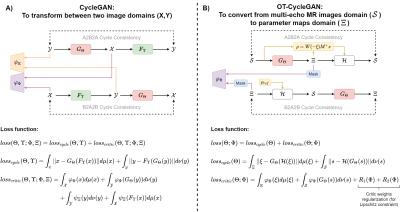
CycleGANs transform samples from one domain into another, using two pairs of neural networks: generator and critic[9]. Both models compete during the training process: while the generator tries to produce realistic synthetic data, the critic tries to discern between generated and real samples, assigning a score according to their verisimilitude. This competition is achieved by an iterative maximization/minimization of a loss function with cycle-consistency and critic terms (Figure 1A).The forward problem is a deterministic physical model whose matrix formulation is defined as follows: $$\mathcal{H}: s(\rho,\xi)=W(\xi)M\rho$$
where $$$s$$$ is a vector with the complex MR signal at each echo, $$$W$$$ is a diagonal matrix that contains the non-linear effects of both $$$R_2^*$$$ and $$$\Delta f$$$ (summarized in the complex variable $$$\xi=\Delta f + i2\pi R_2^*$$$) evaluated at each echo time, $$$M$$$ is a matrix containing the spectral behavior of water and fat, and the $$$\rho$$$ vector contains water ($$$\rho_W$$$) and fat ($$$\rho_F$$$) signals.
Optimal transport theory allows for getting a physics-informed CycleGAN, which dispenses with a generator-critic pair and includes Eq.1 into the loss function (Figure 1B). The goal of the generator is to estimate receiving multi-echo images as input, for a posterior computation of the water-fat signals using the linear least-squares method: $$\rho=W(-\xi)M^+ s$$
Then, $$$PDFF$$$ can be estimated as the ratio of the water and fat signals: $$PDFF=\frac{|\rho_F|}{|\rho_W|+|\rho_F|}$$.
Experiments
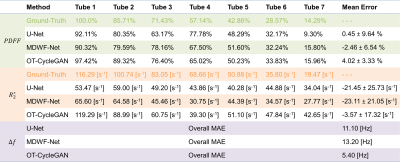
We considered six-echoes 2D multi-slice GRE acquisitions (TE1/ΔTE/TR=1.3/2.1/30 ms) at 1.5T scanner (Achieva, Philips Healthcare) of 158 volunteers. The final database included 3545 slices, that were rescaled to 192x192 pixels by K-space subsampling. Since OT-CycleGAN can be trained using an unpaired dataset, we computed reference results for only 100 patients using Graph Cuts algorithm[4], which we considered as gold standard. The overall slices were divided into training (70%), validation (10%) and testing (20%) subsets.For comparing with other DL approaches, supervised learning U-Net and MDWF-Net models were also trained[7]. Overall mean-absolute-error (MAE) with respect to Graph Cuts results was computed for $$$PDFF$$$, $$$R_2^*$$$ and $$$\Delta f$$$ maps. A one-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test was also performed over $$$PDFF$$$ and $$$R_2^*$$$ estimations at specific liver ROIs (right posterior and left hepatic lobes, RHL and LHL respectively), considering a significance level of 0.05. To assess the ability of generalization, we also considered a simulated phantom that contained 7 tubes with different $$$PDFF$$$ (range: 14-100%) and $$$R_2^*$$$ (range: 19-116[s-1]) values and a $$$\Delta f$$$ map with a linear gradient (range: -300-300[Hz]).
Results
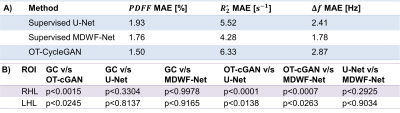
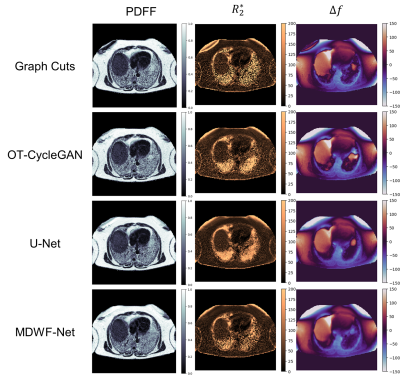
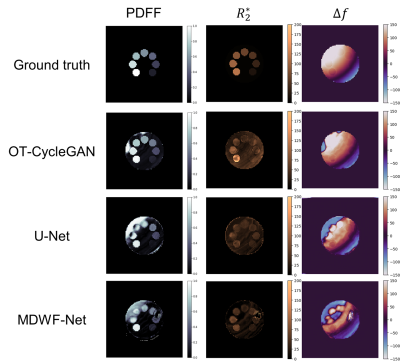
Supervised models showed lower $$$R_2^*$$$ and $$$\Delta f$$$ MAEs with respect to Graph Cuts than OT-CycleGAN. However, the latter displayed a lower $$$PDFF$$$ MAE. (Table 1A). One-way ANOVA showed significant differences for $$$R_2^*$$$ values at both RHL (p<0.0001) and LHL (p=0.0448). Particularly, Bonferroni post hoc test showed statistically significant differences between OT-CycleGAN and all the other methods (Table 1B). However, one-way ANOVA showed no significant differences for $$$PDFF$$$ estimations at RHL (p=0.997) and LHL (p=0.947). Qualitatively, $$$R_2^*$$$ maps were highly denoised compared to Graph Cuts results, and $$$\Delta f$$$ maps showed fewer swapping artifacts (Figure 2).For the synthetic phantom (Figure 3), one-way ANOVA indicated no significant differences between all DL methods at $$$PDFF$$$ (p=0.9938) and $$$R_2^*$$$ (p=0.1996) estimations. However, OT-CycleGAN obtained a lower mean error and standard deviation for $$$R_2^*$$$, and a lower standard deviation for $$$PDFF$$$. For $$$\Delta f$$$ maps, OT-CycleGAN showed the lowest overall MAE (Table 2).
Discussion
OT-CycleGAN overperformed supervised models in $$$PDFF$$$ estimation. Nevertheless, only supervised models showed no significant differences to Graph Cuts in $$$R_2^*$$$ and $$$\Delta f$$$ estimations. However, a qualitative analysis of $$$R_2^*$$$ and $$$\Delta f$$$ suggested that supervised models could have replicated some Graph Cuts artifacts (noisy $$$R_2^*$$$, swaps in $$$\Delta f$$$) that were corrected by OT-CycleGAN.Moreover, phantom’s results showed that OT-CycleGAN significantly overperformed the ability of generalization of supervised DL models.
Conclusion
OT-CycleGAN is a DL approach for the water-fat separation problem that could be trained with a partially labelled dataset and can achieve similarly accurate $$$PDFF$$$ estimations than the gold standard Graph Cuts algorithm. Moreover, due to its ability of generalization, OT-CycleGAN could be a more reliable alternative to use in clinical practice than other DL methods.Acknowledgements
This work was funded by ANID – Millennium Science Initiative Program – NCN17_129. J.M. was funded by the National Agency for Research and Development (ANID) / Scholarship Program / DOCTORADO BECAS CHILE/2020 – 21210665. S.U. was funded by Fondecyt 1181057. C.T. was funded by Fondecyt 1191710, Anillo PIA ACT192064.References
[1] J. Starekova, D. Hernando, P.J. Pickhardt, S.B. Reeder, Quantification of Liver Fat Content with CT and MRI: State of the Art, Radiology. (2021) 204288. https://doi.org/10.1148/radiol.2021204288.
[2] G. Hamilton, T. Yokoo, M. Bydder, I. Cruite, M.E. Schroeder, C.B. Sirlin, M.S. Middleton, In vivo characterization of the liver fat 1H MR spectrum, NMR Biomed. 24 (2011) 784–790. https://doi.org/10.1002/nbm.1622.
[3] S.B. Reeder, Z. Wen, H. Yu, A.R. Pineda, G.E. Gold, M. Markl, N.J. Pelc, Multicoil Dixon Chemical Species Separation with an Iterative Least-Squares Estimation Method, Magn. Reson. Med. 51 (2004) 35–45. https://doi.org/10.1002/mrm.10675.
[4] D. Hernando, P. Kellman, J.P. Haldar, Z.P. Liang, Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm, Magn. Reson. Med. 63 (2010) 79–90. https://doi.org/10.1002/mrm.22177.
[5] J.W. Goldfarb, J. Craft, J.J. Cao, Water–fat separation and parameter mapping in cardiac MRI via deep learning with a convolutional neural network, J. Magn. Reson. Imaging. 50 (2019) 655–665. https://doi.org/10.1002/jmri.26658.
[6] R. Jafari, P. Spincemaille, J. Zhang, T.D. Nguyen, X. Luo, J. Cho, D. Margolis, M.R. Prince, Y. Wang, Deep neural network for water/fat separation: Supervised training, unsupervised training, and no training, Magn. Reson. Med. (2021). https://doi.org/10.1002/mrm.28546.
[7] J.P. Meneses, C. Arrieta, G. della Maggiora, P. Irarrazaval, C. Tejos, M.E. Andia, C. Sing-Long, S. Uribe, Water/fat separation using multiple decoder U-Net architecture (MDWF-Net) for accurate R2* measurements, in: Proc. 29th Annu. Meet. ISMRM, 2021.
[8] B. Sim, G. Oh, J. Kim, C. Jung, J.C. Ye, Optimal Transport, CycleGAN, and Penalized LS for Unsupervised Learning in Inverse Problems, (2019) 1–25. http://arxiv.org/abs/1909.12116.
[9] J.-Y. Zhu, T. Park, P. Isola, A. Efros, Unpaired Image-to-Image Translation using Cycle-Consistent Adversarial Networks Jun-Yan, Proc. IEEE Int. Conf. Comput. Vis. (2017) 183–202. http://link.springer.com/10.1007/978-1-60327-005-2_13.
Figures