2283
Prediction of MCI conversion using sulcal morphometry1Department of Biomedical and NeuroMotor Sciences, University of Bologna, Bologna, Italy, 2Functional and Molecular Neuroimaging Unit, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy, 3Department of Experimental, Diagnostic and Specialty Medicine, University of Bologna, Bologna, Italy, 4Department of Neurosciences "Rita Levi Montalcini", University of Torino, Torino, Italy, 5UOC Clinica Neurologica, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy, 6Department of Physics and Astronomy, University of Bologna, Bologna, Italy
Synopsis
We investigated the capabilities of brain sulcal morphometry and cortical thickness (CT) to predict the conversion from MCI stage to AD and explored the correlation with neuropsychological performance. Sulcal surface, mean depth, length and width were analysed. The width showed the best capability to discriminate between HC, MCI and AD (also in comparison to CT), and was able to distinguish at baseline converter MCI from non-converter MCI. Moreover, the width of specific frontal sulci correlated with memory and language performance.
Sulcal morphometry emerged as a useful tool, capable of providing potential biomarkers for MCI conversion.
Introduction
Mild Cognitive Impairment (MCI) is an intermediate clinical stage between the cognitive decline of aging and the earliest features of dementia1. The outcomes of its progression could vary from Alzheimer’s Disease (AD) or non-AD dementia to the stabilization of symptoms.Previous findings have shown that different neuroimaging techniques can discriminate between healthy controls (HC), MCI and AD2-4 and may be able to predict those MCI that will potentially convert to AD5-7. The main goal of this study was to investigate the ability of sulcal morphometry and cortical thickness (CT) to predict the progression from MCI to AD, based on clinical classification at 2-years follow-up, and to explore the correlations with clinical variables.
Methods
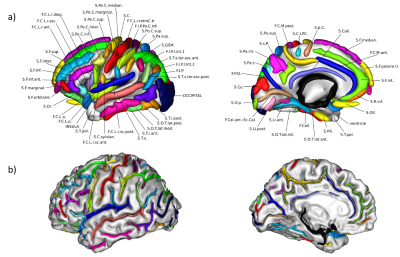
Twenty-five AD patients (15 males, age=70.8±9.1 years), thirty-seven MCI (19 males, age=73.7±7.4 years) and twenty-five HC (11 males, age=68.4±9.2 years) were included in this study. All participants underwent a brain-MR protocol (1.5T GE scanner) including a T1-weighted volumetric sequence (isotropic 1mm3 resolution). MCI patients underwent at baseline an extensive neuropsychological assessment (using memory, language, visuospatial, attention and executive function tests) and were clinically re-evaluated after 28±15 months. At follow-up, 12 MCI were classified as MCI non-converter (MCInc) and 25 as MCI converter (MCIc).Sulcal morphometry was performed using the ENIGMA Sulci protocol8,9 (https://hub.docker.com/r/fpizzaga/sulci), based on the BrainVISA framework (https://brainvisa.info/web/index.html). To obtain reliable and consistent morphometric measures across subjects, the automatic labelling of sulci in each subject was visually inspected and manually changed where needed (Fig 1); finally, four features (surface, mean depth, length and width) of 123 sulci were retrieved. In addition, cortical thickness (CT) was assessed using FreeSurfer (https://surfer.nmr.mgh.harvard.edu/). All data were corrected for the Total Intracranial Volume.
The features of each sulcus were compared among groups of participants using a Kruskal-Wallis test, followed by a pair-wise Bonferroni post-hoc test for multiple comparisons. For each parameter able to discriminate the groups, receiver operating characteristic (ROC) curve analysis was also performed by fitting a logistic regression, to determine specificity, sensitivity, area under curve (AUC) and accuracy. The results obtained for each feature were cross-validated with a Leave-One-Out approach applied recursively on each subject. The same procedure was used for the CT data.
Spearman’s correlation, corrected for multiple comparisons, was used to explore the possible relationships between the sulcal features that successfully discriminated MCIc from MCInc and cognitive scores.
Results
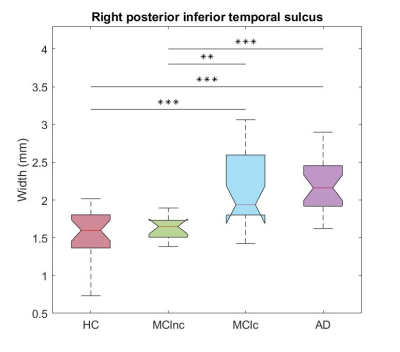
All the sulcal features exhibited significant alterations across different brain areas, although sulcal width showed the best abilities in the differentiation between each pair of groups. For each contrast, the most significant result is reported in the following: the width of the left rhinal sulcus (AUC=0.757) when comparing HC vs MCInc, of the left posterior occipito-temporal lateral sulcus (AUC = 0.941) when comparing HC vs MCIc, of the left collateral fissure (AUC=0.947) when comparing HC vs AD and of the right posterior inferior temporal sulcus when comparing both MCInc vs MCIc and MCInc vs AD (AUC=0.907 and AUC=0.957, respectively, see Fig. 2); no sulcal feature was able to distinguish MCIc from AD.Focusing on the contrast between MCInc and MCIc, additional alterations of width able to distinguish the two subgroups were found in the bilateral temporo-occipital area (e.g., the left occipito-temporal lateral sulcus, AUC=0.853) and in the frontal lobe of the left hemisphere (e.g., the intermediate frontal sulcus, AUC=0.843) (Fig. 3). The CT features were only able to discriminate MCIc and AD from HC and AD from MCInc, reaching generally lower classification performances (e.g., the right inferior temporal cortex achieved AUC=0.884 when comparing HC vs AD).
The cross-validation confirmed the reliability of the in-sample analysis, showing comparable results from the ROC analysis.
In the MCI group, only the sulcal width exhibited significant correlations with specific NPS scores. Specifically, the right olfactory and the left inferior precentral sulci negatively correlated with the immediate Rey Auditory Verbal Learning Test (RAVLT); the left olfactory sulcus negatively correlated with the delayed RAVLT and the right intermediate frontal sulcus with the phonemic fluency.
Discussion
The sulcal features, width in particular, demonstrated significantly better discriminative capabilities with respect to CT, not only by being able to distinguish MCIc from MCInc, but also achieving greater accuracies for each pair-wise comparison.The manual relabelling of sulci had a crucial role in this study, since certain cortical folds, such as the right posterior inferior temporal sulcus, the most significant one when comparing MCIc with MCInc, are not reliably recognized by the software across subjects8.
The sulci showing the best discriminative capabilities across groups were located in the temporal and temporo-occipital areas. In addition, higher width in the left and right olfactory sulci was associated with worse memory performance in MCI patients. Notably, the most discriminative sulci able to distinguish MCIc from MCInc, were also able to distinguish cross-sectionally healthy controls from different pathological stages, indicating that they could be efficient biomarkers for prognosis.
Conclusions
Our results showed that sulcal morphometry is a useful tool, able to discriminate between HC, MCI and AD, especially through the sulcal width. In addition, it exhibited high accuracy in predicting the progression from MCI to AD.Acknowledgements
No acknowledgement found.References
1Albert MS, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270-279. doi: 10.1016/j.jalz.2011.03.008
2Im K, et al. Sulcal morphology changes and their relationship with cortical thickness and gyral white matter volume in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2008;43(1):103-113. doi: 10.1016/j.neuroimage.2008.07.016
3Bertoux M, et al. Sulcal morphology in Alzheimer's disease: an effective marker of diagnosis and cognition. Neurobiology of aging. 2019,84:41-49. doi: 10.1016/j.neurobiolaging.2019.07.015
4Cai K, et al. Identification of Early-Stage Alzheimer's Disease Using Sulcal Morphology and Other Common Neuroimaging Indices. PLOS ONE. 2017;12(1): e0170875. doi: 10.1371/journal.pone.0170875
5E. Ficiarà et al., "Predicting Progression from Mild Cognitive Impairment to Alzheimer’s Disease using MRI-based Cortical Features and a Two-State Markov Model," 2021 IEEE 18th International Symposium on Biomedical Imaging (ISBI), 2021, pp. 1145-1149. doi: 10.1109/ISBI48211.2021.9434143
6Mitolo M, et al. Predicting conversion from mild cognitive impairment to Alzheimer's disease using brain 1H-MRS and volumetric changes: A two- year retrospective follow-up study. Neuroimage: Clinical. 2019;23: 101843. doi: 10.1016/j.nicl.2019.101843
7Plocharski M, Østergaard LR. Prediction of Alzheimer’s disease in mild cognitive impairment using sulcal morphology and cortical thickness. L. Lhotska, L. Sukupova, I. Lacković, & G. S. Ibbott (eds.), World Congress on Medical Physics and Biomedical Engineering. 2018. IFMBE Proceedings. 68(1). Springer, Singapore. doi: 10.1007/978-981-10-9035-6_13
8Pizzagalli F, et al. The reliability and heritability of cortical folds and their genetic correlations across hemispheres. Commun Biol. 2020; 3, 510. doi: 10.1038/s42003-020-01163-1
9Dojat M, et al. Magnetic resonance imaging does not reveal structural alterations in the brain of grapheme-color synesthetes. PLoS One. 2018; 13(4). doi: 10.1371/journal.pone.0194422
Figures