2280
Improved 7T High-Resolution Human Hippocampal Imaging with Multi-Frequency Magnetization Transfer (MFMT)1MRI Research Center, Auburn University, Auburn University, AL, United States
Synopsis
Alzheimer’s disease (AD) and other cognitive debilitating disease drive the need to improve neuro 7T MRI methods. We developed a multi-frequency magnetization transfer (MFMT) method for contrast improvement in 7T 3D MRI of the human hippocampus. MFMT applied to healthy volunteers demonstrated a 2.05 (p < 0.003) factor improvement in hippocampal contrast.
Introduction
An estimated 5 million Americans have suffered from Alzheimer’s disease (AD) since 2010 and are expected to triple by 2050 [1]. AD and Mild Cognitive Impairment (MCI) have been associated with hippocampal atrophy when compared to normal controls. Neurofibrillary tangle deposition and neuronal loss have also been associated with hippocampal atrophy [2]. Ultra-high field 7T MRI is transitioning into clinical neurological use and may become the ideal method to detect and quantify hippocampal atrophy with its 3D high-resolution capability. Simple translation of lower field 3T methods to 7T are insufficient due to higher inhomogeneity effects, SAR restrictions, and need for higher contrast. Specifically for 7T contrast enhancement, we developed an improved Multi-Frequency Magnetization Transfer (MFMT) method to couple with low-SAR 3D acquisition. MFMT was developed and applied to healthy volunteers to quantify the contrast improvement with 7T hippocampal MRI.Methods
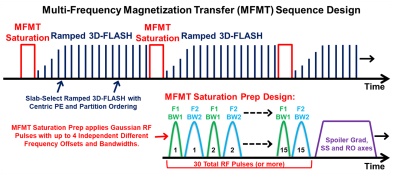
Previous literature reported improved results with standard MT methods optimized at targeted brain locations [3, 4]. These previous methods applied mono-pulse MT saturation at single offset frequencies and bandwidths that best-matched the target ROI biomolecular environment. Our MFMT expands on this to allow multiplexing up to four RF pulses with independent offset frequencies and independent bandwidths – thereby simultaneously saturating up to four biomolecular environments. This allows a highly tailored MT spectrum to be combined with efficient 3D-FLASH image acquisition, as shown in Figure 1. The saturation RF pulse are Gaussian, with RF phase stepping (spoiling) between pulses. As the MFMT induces the contrast in this sequence, the 3D-FLASH allows maximum readout SNR. Preliminary test runs applied MFMT offsets at ±11.8, ±6.7, ±4.0 and ±3.5 ppm to find an optimal combination for best contrast in the hippocampus.Four healthy volunteers, with informed consent, were scanned in a Siemens 7T scanner, using a Nova 1 Tx/32 Rx head coil. A coronal 3D slab was positioned to simultaneously image both hippocampi. The 3D slab/volume image with isometric pixels allowed for easy post-scan reslicing to any cor/sag/tra direction. Scans with MFMT 'on' and 'off' were run to allow comparison. On multiple acquired hippocampal slices, contrast-to-noise (CNR) analysis was performed between the hippocampal sulcus (dark) versus the neighboring CA4 region (bright).
Scan parameters: MFMT: Simultaneous saturation frequencies = ±11.8 and ±6.7 ppm each with bandwidth = 0.84ppm, Gaussian pulses, flip-angle = 120º, 30-pulse saturation train length. 3D-FLASH: Slab-selective FOV = 256x240x24 mm, matrix = 688x645x64, Pixel size = 0.4x0.4x0.4 mm, FA = 7.5º, TE = 3.4 ms, TR = 850 ms, BW = 360 Hz/Pix, Avg = 1, Duration = 7 min, 30 sec.
Results
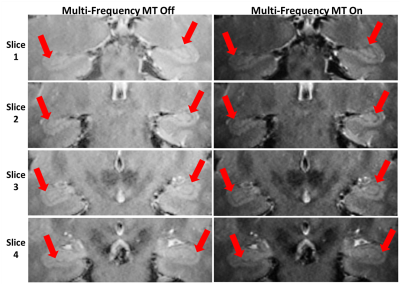
Preliminary scans indicated the best hippocampal anatomical contrast was obtained with simultaneous saturations at offsets +11.8, +6.7, -11.8 and -6.7 ppm, all with bandwidth 0.84ppm. From this preliminary data, in vivo hippocampal 3D MRI sets were acquired from all four volunteers. Figure 2 shows results from one volunteer at four transverse hippocampal slice loacations. The Figure 2 images present a clear increase in contrast for MFMT 'on' versus 'off'. The red arrows indicate the hippocampal regions and the increased contrast between the thin dark hippocampal sulcus and surrounding tissue regions. The improved contrast facilitates the detection and tracking of atrophy. CNR quantification analysis for MFMT 'on' versus 'off' gave a mean CNR increase of 4.43±1.63 (mean±stdev). This 4.43 mean difference is equivalent to a CNR improvement ratio of 2.05 (p < 0.003) -- therefore MFMT effectively “doubled” the contrast.Discussion
The effectively doubled contrast provided discernible anatomical contrast at 0.37 mm resolution for improved investigation of hippocampal condition. Similar to other 7T MRI, poor B0 shimming reduced the MFMT contrast effectiveness. Typical SAR levels were low and easily managed by lowering MFMT RF pulse(s) flip angle or increasing sequence repetition time (TR).Conclusions
The applied MFMT demonstrates an effective contrast enhancement method for 7T hippocampal MRI. Continued study will investigate additional offset frequency and bandwidth combinations that further improve hippocampal and other brain region contrast.Acknowledgements
Special thank you for project support goes to Julie Rodiek, Steven Nichols and Adam Davila.References
1. Goukasian N, Porat S, et al “Cognitive Correlates of Hippocampal Atrophy…”, Dement Geriatr Cogn Disord Extra 2019;9:281–293
2. Zarow C, Wang L, et al "MRI Shows More Severe Hippocampal Atrophy…", Inter J of Alzheimer’s Disease, vol. 2011, Article ID 483972, 2011
3. Mougina O, Clemence M, et al “High-resolution imaging of magnetisation transfer…”, NMR Biomed. 2013;26:1508–1517
4. Priovoulos N, Jacobs H, et al “High-resolution in vivo imaging of human locus coeruleus…”, NeuroImage 2018;168:427–436
Figures