2274
7T resting state connectivity applied to HIFU procedures for planning and efficacy1Imaging Institute, Cleveland Clinic, Cleveland, OH, United States, 2Neurosurgery, Cleveland Clinic, Cleveland, OH, United States
Synopsis
High Intensity Focused Ultrasound (HIFU) in now entering clinical practice, for example to treat essential tremor by causing small lesions in the thalamus. Due to small size of treatment lesions, treatment success depends critically on targeting, which is classically done using measurements and landmarks. We explore an alternative method using functional imaging to guide targeting, specifically using 7T resting state connectivity. We present preliminary data of the patterns of connectivity possible with 7T using a concatenated series of 18 healthy subjects.
Introduction
High Intensity Focused Ultrasound (HIFU) is a recently approved treatment for essential tremor (ET) using an MR guided array of 1024 transducers1, which transmit phase-adjusted extracranial ultrasound at 650 kHz to a focal spot in the ventral intermediate nucleus of the thalamus (ViM), to create a permanent thermoablative lesion. The thermal cavity is spherical with a diameter of about 6 mm. This size is similar to the ViM, around 5x7 mm. While maximal clinical benefit occurs with strong overlap of the lesion with ViM, extension into adjacent thalamic nuclei can cause unwanted sided effects, such as ataxia or pain. To date, positioning of the target is guided by using brain measurements, which is indirect, for example using locations of the anterior and posterior commissures, which are fairly constant among all adults. Alternative methods use direct targeting using structural imaging to visualize thalamic nuclei. However, these methods are often insufficient, and there is great utility in providing direct targeting with functional imaging. We propose to use a dataset of concatenated 7T resting state fMRI to address the unmet problem of directly imaging the ViM target using a functional technique.Methods
Eighteen healthy control subjects were scanned, under an IRB-approved protocol, on a Magnetom 7T equipped with a SC72 gradient coil (Siemens Healthineers, Erlangen) using a 32-channel head coil (Nova Medical). Sequences included a whole-brain anatomical MP2RAGE (T1w, 0.75 mm isotropic voxels), and resting state-fMRI. Rs-fMRI acquisition parameters included between 118 to 128 repetitions of 81 1.5mm thick axial slices acquired with TE/TR=19ms/2800ms, matrix 160x160, FOV 210mm x 210mm, receive bandwidth = 1562 Hz/pixel, MB=3, Grappa=2. Subjects were instructed to keep their eyes closed during scans and refrain from any motion.Data Pre-Processing: Each rsfMRI dataset was corrected to remove physiologic noise2 and head motion3, and B0 distortions. The anatomical scans were then nonlinearly registered to a modified MNI template. Then rsfMRI data were warped to the template in a single step using Bspline interpolation using ANTS. All rsfMRI were concatenated to one large dataset using 3dTcat4, yielding a time series with about 2300 volumes. The resulting rsfMRi dataset was input into AFNI’s Instacor using the following pre-processing: 2mm FWHM Gaussian smoothing, quadratic detrending, and temporal filter with passband 0.01 - 0.1 Hz, L2-normed. ·
Functional Connectivity (FC) Analysis: An ROI was defined as a rectangular cube that encompassed the bilateral thalamus, extending superiorly to the lateral ventricles, and inferiorly to the pons, with 1 mm cubic voxel size. A cortical parcellation was first generated from FreeSurfer using the MP2RAGE for each subject. Each cortical parcel was dilated by 1 mm to help maximize overlap in the set of all patients. Each voxel in the ROI was used as a seed to produce functional connectivity (FC) maps between that voxel and all gray matter voxels, using a seed radius of 2 mm. The resulting Student’s t maps were corrected for mean and standard deviation to yield z-score maps3,5. A threshold was applied to all Z-maps: z=2.0 for positive maps, and -2.0 for negative maps. An additional cluster threshold of 30 voxels was applied. Lastly, for each parcel in the FreeSurfer parcellation, a final connectivity score was computed as the total number of voxels within that parcel that survived the thresholds and clustering. This process was repeated for all voxels in the thalamic ROI, which represented 170,982 voxels. This computation initially was performed using code written in IDL that called Instacorr on a server with 56 CPUs and 256 GB RAM. This was subsequently improved using a single MATLAB code with direct memory mapping techniques.
Results
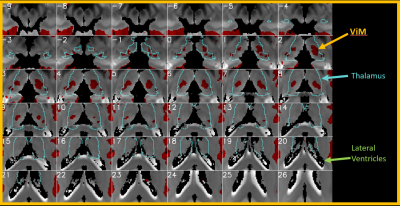
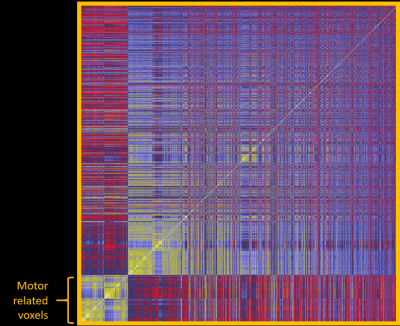
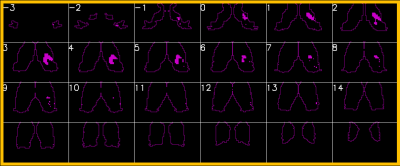
The entire computation initially took 12 days to complete, which was later reduced to 2 days after implementation of the memory mapping technique. Figure 1 shows one example of connectivity from voxels in the thalamic ROI to motor cortices (defined as FreeSurfer parcels from aparc2009 comprising central sulcus (gyral crown and anterior sulcal bank), precentral, paracentral, and subcentral gyri). Numerous other connectivity maps are possible, including those to limbic, visual, and frontal circuits. Small nuclei can be visualized outside the thalamus, for example median raphe nucleus (not shown). An additional analysis is a data-driven parcellation of the entire thalamus using clustering algorithms, based on its connectivity patterns to cortex. Figure 2 shows an example of the connectivity matrix for left thalamic voxels (7115 voxels total) to left hemispheric gray matter parcels. The clusters clearly noted at the bottom left corner are those associated with motor function in the thalamus. Figure 3 shows those voxels in the thalamic map that corresponds to that cluster, and this coincides with the known morphology of ViM.Discussion and Conclusion
High spatial-resolution 7T resting state connectivity from a large concatenated dataset from numerous normal healthy controls provides an accurate way to study connectivity of thalamic nuclei. The goal of such maps to develop and verify pattern templates of motor-thalamic connectivity, and thereby reliable identify ViM in individual patients, for direct targeting for HIFU procedures.Acknowledgements
The authors acknowledge Anna Crawford, M.S. for assistance creation of the concatenated rsfMRI dataset used in this work. We also acknowledge Tobias Kober and Thomas Benner from Siemens Healthineers, Inc. for use of the MP2RAGE sequence and multi-band EPI, respectively.References
1. Elias, W.J., Lipsman, N., Ondo, W.G., Ghanouni, P., Kim, Y.G., Lee, W., Schwartz, M., Hynynen, K., Lozano, A.M., Shah, B.B., Huss, D., Dallapiazza, R.F., Gwinn, R., Witt, J., Ro, S., Eisenberg, H.M., Fishman, P.S., Gandhi, D., Halpern, C.H., Chuang, R., Butts Pauly, K., Tierney, T.S., Hayes, M.T., Cosgrove, G.R., Yamaguchi, T., Abe, K., Taira, T.,Chang, J.W. 2016. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N Engl J Med 375, 730-739.
2. Beall EB, Lowe MJ. Isolating physiologic noise sources with independently determined spatial measures. Neuroimage. 2007 Oct 1;37(4):1286-300.
3. Beall EB, Lowe MJ. SimPACE: generating simulated motion corrupted BOLD data with synthetic-navigated acquisition for the development and evaluation of SLOMOCO: a new, highly effective slicewise motion correction. Neuroimage. 2014 Nov 1;101:21-34.
4. Lowe MJ, Crawford A. Improved Specificity of Functional Mapping of Thalamocortical Connections at Ultra High Field. Proc 30th ISMRM 2020; #3972.
5. Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998 Feb;7(2):119-32.
Figures