2273
Automated 3D MRI Decision Tree to guide referral and diagnosis of parkinsonian disorders1Neuroradiology, Brigham and Women's Hospital, Boston, MA, United States, 2Movement Disorders Division, Neurology, Brigham and Women's Hospital, Boston, MA, United States, 3Neurology, Massachusetts General Hospital, Boston, MA, United States, 4Center for Clinical Investigation, Brigham and Women's Hospital, Boston, MA, United States, 5Diagnostic Radiology, Brigham and Women's Hospital, Boston, MA, United States, 6Imaging, Dana Farber Cancer Institute, Boston, MA, United States
Synopsis
For screening and diagnosis of Parkinson disease (PD), progressive supranuclear palsy (PSP), and multiple system atrophy-parkinsonian/cerebellar subtypes (MSA-P/MSA-C), we propose a two-step decision tree using a novel MRI biomarker and volumetric measures. MRIs from 50 MSA-C, 22 MSA-P, 50 PSP, 50 PD, and 172 age/legal-sex-matched control were used for fully automated volumetric analysis of the brain and brainstem. Sensitivity/specificity for diseased vs control was 90.1/90.7%. Then, a comprehensive tree with the diseased group yielded sensitivity/specificity of 81.9/93.0% (MSA), 94.0/88.5% (MSA-C), 54.5/96.0% (MSA-P), 74.0/86.0% (PD), 76.0/98.4% (PSP). We provide a reproducible and specific diagnostic tool for screening and initial differential diagnosis.
Introduction
Differentiation of parkinsonian disorders such as Parkinson disease (PD), progressive supranuclear palsy (PSP), and multiple system atrophy (MSA) has been challenging, not to mention further categorization of MSA patients into parkinsonian and cerebellar subtypes (MSA-P/C) which has clinically significant implications regarding early therapy and management. Previously published MRI biomarkers have been used to help differentiate these, but have not been packaged into a fully automated, straight-forward clinical decision-making algorithms that target heterogeneous populations encountered in the clinic including healthy population as well as all the above diagnoses.1,2 We propose a comprehensive decision-making tool to guide the generalist clinician in triaging referral to specialists as well as aid movement specialists.Methods
T1-weighted volumetric MRI of 50 MSA-C (13 autopsy-confirmed, 37 probable), 22 MSA-P (4 autopsy-confirmed, 18 probable), 50 PSP (7 autopsy-confirmed, 43 probable), and 50 PD (9 autopsy-confirmed, 41 probable), and 172 age and legal-sex-matched control patients were automatically segmented with Freesurfer 6.0 and its brainstem module. Volumes of brain and brainstem structures and a newly created variable which is pons volume to midbrain volume ratio (3D-PMR) were used to create decision trees using Rpart package in R, followed by 10-fold 10-repeat cross-validation with Caret package.Results
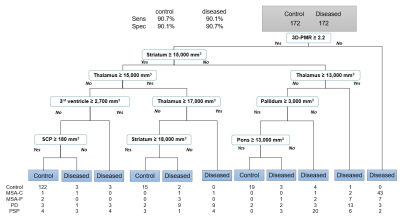
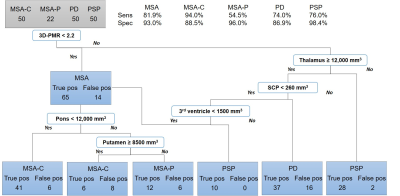
A screening tree to filter control patients selected 3D-PMR, striatum, thalamus, third ventricle, pallidum, and pons, and superior cerebellar peduncle (SCP) volumes as nodes, and demonstrated sensitivity of 90.1% and specificity of 90.7% for the diseased group with 82.4% accuracy and 0.65 kappa from cross-validation. Then, a comprehensive tree with the diseased group used 3D-PMR, thalamus, SCP, third ventricle, pons, and putamen as its nodes, and yielded sensitivity/specificity of 81.9/93.0% (MSA), 94.0/88.5% (MSA-C), 54.5/96.0% (MSA-P), 74.0/86.0% (PD), 76.0/98.4% (PSP). Cross-validation showed 78.0% accuracy and 0.61 kappa. This tree correctly classified 15/17 MSA, 5/7 PSP, 7/9 PD autopsy-confirmed patients.Discussion
The screening tree suggested lower volumes – likely from atrophy – of the striatum, pallidum, pons, and SCP helps differentiate the diseased group from the control. However, higher volumes of thalamus led to the diseased group which may be due to previously reported enlarged thalamus seen in patients with Parkinson disease.3 3D-PMR was also helpful in differentiating the diseased from control. To differentiate the diseased, a lower 3D-PMR suggested the diagnosis of MSA. Furthermore, pons atrophy suggested MSA-C while putamen atrophy suggested MSA-P. Thalamus atrophy suggested PSP.Conclusion
Volumetric and decision tree analysis confirms the generally accepted pathophysiology behind the studied atypical parkinsonian diseases. Our study further provides specific volumetric and variable (3D-PMR) values that can aid in decision making in the clinic. Our approach differs from the past in terms of reliability of its sample and method, complete automation including brainstem segmentation, inclusion of pathologically-confirmed patients within each cohort, and consideration of heterogeneity of clinic population by including a healthy control group. This approach builds on past work to provide a reproducible and highly specific diagnostic tool for screening and initial differential diagnosis of atypical neurodegenerative movement disorders.Acknowledgements
Grant funding and support was provided by the Multiple System Atrophy Coalition (V.K.) and Barbara Bloom Ranson Fund for Biomarker Discovery in MSA (M.M., V.K). V.K. is a New York Stem Cell Foundation – Robertson Investigator. X.X was supported by NIH award R01LM012434.References
1. Scherfler C, Gobel G, Muller C, et al. Diagnostic potential of automated subcortical volume segmentation in atypical parkinsonism. Neurology. 2016;86(13):1242-1249.
2. Chougar L, Pyatigorskaya N, Degos B, Grabli D, Lehericy S. The Role of Magnetic Resonance Imaging for the Diagnosis of Atypical Parkinsonism. Front Neurol. 2020;11:665.
3. D'Cruz N, Vervoort G, Chalavi S, Dijkstra BW, Gilat M, Nieuwboer A. Thalamic morphology predicts the onset of freezing of gait in Parkinson's disease. NPJ Parkinsons Dis. 2021;7(1):20.
Figures