2272
Structural and functional correlates of disinhibited behaviors in Essential Tremor1Neurology, Vanderbilt University, Nashville, TN, United States
Synopsis
Emerging evidence has implicated the cerebellum in not just motor, but cognitive and behavioral roles in the brain. Previous research in cerebellar diseases have reported impulsive behaviors, but it is unknown if Essential Tremor (ET) patients present with these behaviors and where they localize. Here, in 105 ET patients, we measured disinhibition using the Frontal Systems Behavior Scale (FrSBe) and assessed the relationship between disinhibition scores and grey matter volume in the cerebellum, as well as functional correlates of disinhibition-related grey matter atrophy. These results are the first to evaluate neurobiological changes associated with disinhibition in ET.
Background
Essential Tremor (ET) is a motor movement disorder that involves cerebellar atrophy and dysfunction of cerebellar networks. Recently, non-motor features of ET have been recognized, such as cognitive impairment, depression, and anxiety1–3. Impulsivity is reported in other cerebellar diseases4,5 but it remains unknown whether impulsivity occurs in ET and is related to cerebellar atrophy. Impulsivity in other diseases like Parkinson disease is linked to ventral-striatal and mesolimbic pathophysiological changes6–8. The cerebellum is hypothesized to have a modulatory effect on cortical circuits. One method of testing this hypothesis is to measure the functional relationship between cerebellar regions and cerebral regions using resting state functional connectivity. In the current study, we test the hypotheses that impulsivity occurs in ET, is related to cerebellar atrophy, and that the locations of cerebellar atrophy occur in regions functionally anti-correlated to mesolimbic regions in the orbitofrontal cortex, anterior cingulate cortex, and medial prefrontal cortex.Methods
Participants: 105 Essential Tremor (ET) patients were consented and scanned at 3T (Achieva, Philips Healthcare) using dual channel body coil transmission and 32-channel phased array reception. The Frontal Systems Behavioral exam (FrSBE) was completed by caregivers, where consideration of the patient’s behavior was assessed. FrSBe scores were normalized by age, sex, and years of education9. Demographic data are described in Table 1.Image Acquisition: Scanning included a 3D structural T1-weighted whole brain image, (MPRAGE, TR/TE=8.9/4.6 ms; turbo gradient echo factor=131; spatial resolution=1x1x1 mm3). The functional imaging data (fMRI) were acquired in a separate population of 1,000 healthy adult subjects10. fMRI data were acquired on a 3T MRI scanner (Siemens, Germany) with a 12-channel phased-array head coil using a gradient-echo echo-planar imaging (EPI) sequence sensitive to blood oxygenation level-dependent (BOLD) contrast (TR/TE= 3,000/30 ms; flip angle = 85°, FOV = 216, spatial resolution = 3x3x3 mm3).
Image analyses: MRI data were processed using Statistical Parametric Mapping 12 software (http://www.fil.ion.ucl.ac.uk/spm/), to evaluate structural properties and associations with FrSBe behavioral scores. Each subject’s T1-weighted scan was segmented into grey matter (GM), white matter (WM), and cerebrospinal fluid (CSF) and then linearly and non-linearly warped to PD25-MNI space (http://stnava.github.io/ANTs/). To evaluate differences in GM volume in relation to disinhibition scores, we performed a general linear model within the GM volumes of the entire cerebellum controlling for age and sex. Parametric statistical maps were evaluated using a cluster size threshold of 25 voxels and p-value corrected (p < 0.05) using the false discovery rate (FDR).
We then quantitatively tested whether disinhibition-related cerebellar atrophy was functionally connected to cerebral regions previously described to be involved in impulsive/disinhibition-like behaviors8,11–13. Specifically, we used our resting state functional connectivity dataset to compute the temporal correlation between spontaneous brain activity recorded from the p-corrected significant grey matter region-of-interest from our VBM results. First, VBM atrophy results in PD25-MNI space were registered to MNI152 space using FSL FLIRT and FNIRT14,15. Using a publicly available normative functional connectivity dataset of 1,000 healthy volunteers from the Brain Genomics Superstruct Project (GSP)10, we measured average blood-oxygenation-level-dependent (BOLD) time-courses within the seed region and correlated these values with the BOLD time course at every other brain voxel. Resulting r-values were converted to a normal distribution using Fischer’s r-to-z transform and were used to compute a single-group, voxel-wise t-test across the 1,000 subjects in the normative dataset to generate network t-maps. To visualize these maps, we thresholded and binarized each map at t > 20, corresponding to a voxel-wise family-wise error corrected p-value < 0.05.
Results
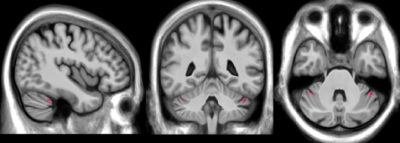
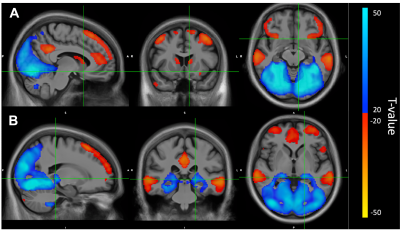
ET patients show clinically significant elevated rates of disinhibition (t-score ≥ 65; Table 1). Cerebellar voxel-based morphometry results show significant decreases in grey matter bilaterally in Crus I16, FDR corrected p = 0.04 (Figure 1). BOLD connectivity results show significant positive correlations with the VBM-based seed region, bilaterally in the cerebellar lobule VIIIb and IX, occipital cortex, sensory cortex, and pulvinar nucleus of the thalamus (Fig. 2). Further, significant anti-correlations with the VBM-based seed region were noted between Crus I of the cerebellum, and the medial frontal lobe, anterior cingulate cortex, inferior frontal gyrus, lateral orbitofrontal cortex, temporal cortex, parietal cortex, precuneus, and caudate (Fig 2.)Discussion
We find that in ET, disinhibition scores are significantly clinically elevated. We also find that these disinhibition scores correlate to decreases in grey matter in Crus I area of the cerebellum, which has been described to be involved in attention and emotional processing17,18. Positive BOLD connectivity associations in occipital and parietal cortex may reflect networks involved in allocation of attention, which have been shown to simultaneously shift oscillations from high-frequency gamma-band to beta- and alpha-band frequencies when switching from attending to unattending a stimulus19. Furthermore, disinhibition-related grey matter atrophy in Crus I of the cerebellum are functionally anti-correlated to previously established areas associated with impulsive behavior, specifically: lateral orbitofrontal cortex, medial prefrontal cortex, anterior cingulate cortex, precuneus, and caudate20,21, suggesting the lateral Crus I area of the cerebellum plays an important role in regulating cortical networks involved in disinhibition. To our knowledge, these results are the first to explore neurobiological underpinnings of disinhibited behaviors in an Essential Tremor cohort and should be considered when determining a treatment plan for this population.Acknowledgements
No acknowledgement found.References
1. Chatterjee A, Jurewicz EC, Applegate LM, Louis ED. Personality in essential tremor: Further evidence of non-motor manifestations of the disease. J Neurol Neurosurg Psychiatry. 2004;75(7):958-961. doi:10.1136/jnnp.2004.037176
2. Thenganatt MA, Louis ED. Personality profile in essential tremor: A case-control study. Park Relat Disord. 2012;18(9):1042-1044. doi:10.1016/j.parkreldis.2012.05.015
3. Cernera S, Okun MS, Gunduz A. A Review of Cognitive Outcomes Across Movement Disorder Patients Undergoing Deep Brain Stimulation. Front Neurol. 2019;10:419. doi:10.3389/fneur.2019.00419
4. Kim JH, Kim TH, Choi YC, Chung SC, Moon SW. Impulsive behavior and recurrent major depression associated with dandy-walker variant. Psychiatry Investig. 2013;10(3):303-305. doi:10.4306/pi.2013.10.3.303
5. Amokrane N, Viswanathan A, Freedman S, et al. Impulsivity in Cerebellar Ataxias: Testing the Cerebellar Reward Hypothesis in Humans. Mov Disord. 2020;35(8):1491-1493. doi:10.1002/mds.28121
6. Claassen DO, Stark AJ, Spears CA, et al. Mesocorticolimbic hemodynamic response in Parkinson’s disease patients with compulsive behaviors. Mov Disord. 2017;32(11):1574-1583. doi:10.1002/mds.27047
7. Ruitenberg MFL, Wu T, Averbeck BB, Chou KL, Koppelmans V, Seidler RD. Impulsivity in Parkinson’s Disease Is Associated With Alterations in Affective and Sensorimotor Striatal Networks. Front Neurol. 2018;9:26. doi:10.3389/fneur.2018.00279
8. Hammes J, Theis H, Giehl K, et al. Dopamine metabolism of the nucleus accumbens and fronto-striatal connectivity modulate impulse control. Brain. 2019;142(3):733-743. doi:10.1093/brain/awz007
9. Grace J, Malloy PH. Outcome Measure Frontal Systems Behavior Scale (FrSBe).; 2001.
10. Buckner RL, Roffman JL, Smoller JW. Brain Genomics Superstruct Project (GSP). 10th ed. Harvard Dataverse; 2014. doi:10.7910/DVN/25833
11. Stark AJ, Smith CT, Lin Y-C, et al. Nigrostriatal and Mesolimbic D2/3 Receptor Expression in Parkinson’s Disease Patients with Compulsive Reward-Driven Behaviors. J Neurosci. 2018;38(13):3230-3239. doi:10.1523/JNEUROSCI.3082-17.2018
12. Ruitenberg MFL, Wu T, Averbeck BB, Chou KL, Koppelmans V, Seidler RD. Impulsivity in Parkinson’s Disease Is Associated With Alterations in Affective and Sensorimotor Striatal Networks. Front Neurol. 2018;9:279. doi:10.3389/fneur.2018.00279
13. Marín-Lahoz J, Martínez-Horta S, Sampedro F, et al. Measuring impulsivity in Parkinson’s disease: a correlational and structural neuroimaging study using different tests. Eur J Neurol. 2020;27(8):1478-1486. doi:10.1111/ene.14235
14. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(SUPPL. 1):208-219. doi:10.1016/j.neuroimage.2004.07.051
15. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782-790. doi:10.1016/j.neuroimage.2011.09.015
16. Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46(1):39-46. doi:10.1016/j.neuroimage.2009.01.045
17. Bernard JA, Seidler RD, Hassevoort KM, et al. Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front Neuroanat. 2012;6(AUG 2012):31. doi:10.3389/fnana.2012.00031
18. Habas C. Functional Connectivity of the Cognitive Cerebellum. Front Syst Neurosci. 2021;15(April):1-8. doi:10.3389/fnsys.2021.642225
19. Friese U, Daume J, Göschl F, König P, Wang P, Engel AK. Oscillatory brain activity during multisensory attention reflects activation, disinhibition, and cognitive control. Sci Rep. 2016;6(March):1-11. doi:10.1038/srep32775
20. Zhao J, Tomasi D, Wiers CE, et al. Correlation between Traits of Emotion-Based Impulsivity and Intrinsic Default-Mode Network Activity. Neural Plast. 2017;2017. doi:10.1155/2017/9297621
21. Gentili C, Vanello N, Podina I, et al. You do not have to act to be impulsive: Brain resting-state activity predicts performance and impulsivity on the Balloon Analogue Risk Task. Behav Brain Res. 2020;379(November 2019):112395. doi:10.1016/j.bbr.2019.112395
Figures