2271
Diffusion MRI Connectometry with Motor and Cognitive Deficits of Pedunculopontine Nucleus Cholinergic Projections in Parkinson’s Disease1Diagnostic Radiology, Singapore General Hospital, Singapore, Singapore, 2Graduate Institution of Medicine, Yuan Ze University, Taiwan, Taiwan, 3Department of Neurology, National Neuroscience Institute, Singapore, Singapore, 4Neurology, National Neuroscience Institute, Singapore, Singapore
Synopsis
Altered brain cholinergic activity has gained attention in Parkinson’s disease (PD). However, the effects of cholinergic dysregulation on motor and cognitive functions remain unclear. This is partly contributed by the limited capability of conventional MRI techniques to detect microstructural changes in the deep brain. We evaluated the cholinergic projections in 36 PD patients and 58 asymptomatic healthy controls using diffusion MRI connectometry. Local diffusion changes in the cholinergic projections showed significant correlations to motor onset, and clinical motor scores in PD compared to control subjects. Our results suggest underlying neuronal inflammation and compensatory mechanisms in early PD-related neural dysfunction.
Introduction
Twenty percent of PD patients who suffer from gait deficit and postural instability are non-responsive to L-DOPA treatment 1,2. This has been attributed to decreased acetylcholine metabolism 3. Considerable evidence for altered cholinergic neurotransmission in PD 4 had emerged using MR tractography and PET tracer techniques to elucidate the underlying neuropathophysiology of PD 1,2,4. The cholinergic neurotransmitter system has a widespread influence on crucial functions such as cognition, attention, gait and postural stability5. The brainstem pedunculopontine nucleus (PPN) is one of the major hubs of cholinergic neuronal projections in the CNS 2. Unfortunately, the effects of cholinergic neurotransmission on the different clinical functions remain unclear in PD. MR studies on the structural connectivity of the cholinergic projections and their correlations with clinical symptoms are scarce. We hypothesize that diffusion MRI connectometry is sensitive to microstructural changes in the cholinergic PPN projections in PD 6-8. We investigated the local connectivity of cholinergic PPN projections in relation to motor and cognitive deficits in PD using diffusion MRI connectometry to gain further insights into the underlying pathophyisology.Method
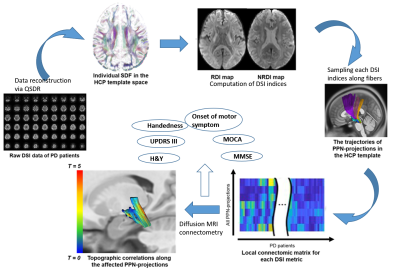
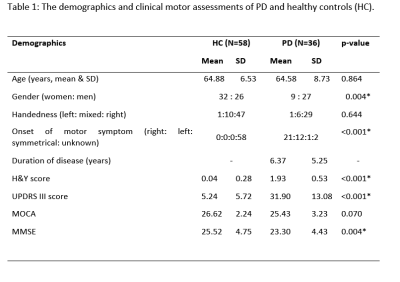
Institutional ethics board approval and patient informed consent were obtained for this study. Ninety-four subjects comprising 36 PD patients and 58 healthy controls (HC) underwent brain MRI using diffusion spectrum imaging (DSI) on a 3T scanner (Skyra, Siemens Healthcare, Erlangen, Germany). The DSI imaging parameters were as follows: TR/TE=4100/110 ms, in-plane resolution = 2x2x2 mm3, diffusion sampling= 129 directions and maximum b-value = 3000 s/mm2. Clinical motor and cognitive assessments including Hoehn and Yahr staging (H&Y), the Unified Parkinson’s Disease Rating Scale part 3 (UPDRS-III), Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) were conducted in all participants. Deterministic fibre tractography was performed using DSI studio (http://dsi-studio.labsolver.org). Cholinergic projections were tracked in both hemispheres (Figure 1). Tracking was run with PPN as a common seed and specific ROI targets as reported in the literature. DSI data were normalised to the Human-Connectome-Project diffusion MRI template and diffusion indices comprising of the quantitative anisotropy (QA), and restricted (RDI) and non-restricted (NRDI) diffusion indices were obtained from the cholinergic tracts. The Kruskall-Wallis test was carried out to establish group differences in the diffusion indices of the PPN projections between PD and HC groups. Further evaluation for voxel-by-voxel correlation to the motor and cognitive assessments was conducted using diffusion MRI connectometry.Results
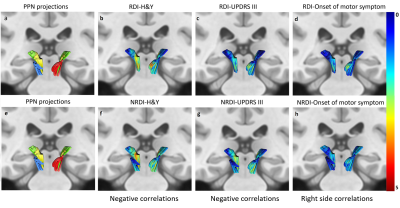
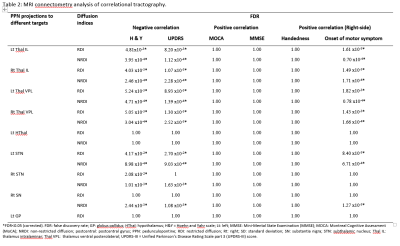
The subject demographics are tabulated in Figure 2. Significant differences were demonstrated in RDI, NRDI, and QA for PPN projections to the thalamus and basal ganglia between PD and HC groups. Diffusion MRI connectometry analysis revealed significant correlations (false discovery rate (FDR) <0.05) only in RDI and NRDI with negative correlations to motor scores (H&Y and UPDRS-III) and right-sided onset of motor symptom between the two groups (Figure 3&4). The affected PPN projections targeted bilateral thalamic intralaminar nuclei, bilateral thalamic ventral posterolateral nuclei, bilateral subthalamic nucleus, and right substantia nigra. Decreased RDI and NRDI were seen with increased H&Y and UPDRS-III scores. The PD group with right-sided presenting motor onset correlated with decreased RDI and NRDI compared to PD with left-sided presenting motor onset and controls without presentation of motor onset. However, no significant correlations were seen between diffusion indices with handedness and cognitive assessments (MOCA & MMSE).Discussion
Comparison of the local connectivity of the PPN cholinergic projections between PD and HC participants showed subtle changes in cholinergic neurotransmission. Further MRI connectometry analysis revealed that decreased RDI and NRDI were significantly correlated with increased H&Y and UPDRS-III motor scores, and right-sided motor symptom presentations in the cholinergic PPN projections in PD patients compared to HC. The local changes in the cholinergic neurotransmission could be driven by several factors such as axonal degeneration, astrocyte reactivity and neuroinflammation, representing early and sustained attempts to compensate for PD-related dysfunction or alteration of acetylcholinesterase in non-neuronal cells 9-11.Conclusion
Diffusion MRI connectometry is a sensitive and refined higher order tractography method that is robust in localizing the segmental WM tract that exhibits significant association with the variable of interest. The subtle local connectivity change of cholinergic PPN projections to the thalamus and basal ganglia network demonstrated significant negative correlations with motor scores and right-sided motor symptom presentations between PD and HC. The cholinergic changes suggest underlying neuroinflammation and compensatory mechanisms for PD-related neural dysfunction.Acknowledgements
We express our appreciation to team of MR Radiographers and research assistants in the Department of Diagnostic Radiology, Singapore General Hospital for their kind assistance and excellent support in this study.References
1. Bohnen NI, Müller ML, Koeppe RA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73(20):1670-1676.
2. Bohnen NI, Kanel P, Zhou Z, et al. Cholinergic system changes of falls and freezing of gait in Parkinson's disease. Ann Neurol. 2019;85(4):538-549.
3. Giladi N, Treves TA, Simon ES, et al. Freezing of gait in patients with advanced Parkinson's disease. Journal of neural transmission (Vienna, Austria : 1996). 2001;108(1):53-61.
4. Müller MLTM, Bohnen NI. Cholinergic dysfunction in Parkinson's disease. Curr Neurol Neurosci Rep. 2013;13(9):377-377. 5. Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2011;26(14):2496-2503.
6. Yeh FC, Tang PF, Tseng WY. Diffusion MRI connectometry automatically reveals affected fiber pathways in individuals with chronic stroke. NeuroImage Clinical. 2013;2:912-921.
7. Yeh FC, Wedeen VJ, Tseng WY. Generalized q-sampling imaging. IEEE Trans Med Imaging. 2010;29(9):1626-1635.
8. Yeh FC, Badre D, Verstynen T. Connectometry: A statistical approach harnessing the analytical potential of the local connectome. NeuroImage. 2016;125:162-171.
9. Yeh FC, Liu L, Hitchens TK, Wu YL. Mapping immune cell infiltration using restricted diffusion MRI. Magnetic resonance in medicine. 2017;77(2):603-612.
10. Chambers NE, Lanza K, Bishop C. Pedunculopontine Nucleus Degeneration Contributes to Both Motor and Non-Motor Symptoms of Parkinson's Disease. Front Pharmacol. 2020;10:1494-1494.
11. Wang N, Zhang J, Cofer G, et al. Neurite orientation dispersion and density imaging of mouse brain microstructure. Brain structure & function. 2019;224(5):1797-1813.
Figures