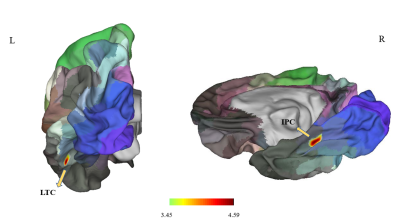
2268
Brain Surface Area Alterations Correlate with Gait Impairments in Parkinson’s Disease1Department of Radiology, Beijing Friendship Hospital, Capital Medical University, Beijing, China, 2MR Collaboration, Siemens Healthcare Ltd., Beijing, China
Synopsis
In this study, we used surface-based morphometry analysis to evaluate the correlation between structural alterations in the brains of Parkinson’s disease (PD) subjects, impaired cognition, and gait. Our results showed that cortical surface area in the left lateral temporal cortex (LTC) and right inferior parietal cortex (IPC) was significantly larger in the PD subjects compared to the healthy subjects. Furthermore, these cortical structural changes were associated with gait impairments in the PD subjects. Therefore, surface area-based analysis is a useful tool for assessing progression of gait impairments in PD subjects.
Introduction
Parkinson's disease (PD) is a common age-related neurodegenerative disease associated with progressive gait, cognitive impairment, and overall functional decline. PD pathology affects multiple neural circuitries and involve structures in the brainstem, basal ganglia, thalamus, subcortex and cortex [1]. Although gait and balance impairments are the most common motor symptoms of PD, the pathophysiology underlying gait, including freezing of gait (FOG), is not fully understood. Cognitive decline is the most frequent non-motor symptom of PD [2, 3]. The relationship between gait and cognitive decline is well documented in PD subjects. In PD, progressive decline in cognition and mobility is associated with falls and other gait-related symptoms [4]. Abnormal gait and balance can produce frozen gait, which will lead to falls, which is the main reason for loss of life independence and increased mortality. In this study, we used surface-based morphometry to determine structural alterations in the brains of PD subjects. We then evaluated the association between structural alterations in the brain, cognitive behavioral measures, and gait impairments in PD patients.Materials and Methods
We included 27 PD patients and 37 healthy controls in this study. The MAGNETOM Prisma 3T MRI system (Siemens, Erlangen, Germany) with a 64-channel phase-array head coil was used to acquire high-resolution 3D structural T1-weighted images with the 3D magnetization-prepared rapid gradient echo (MPRAGE) sequence. The parameters of the MPRAGE sequence were as follows: repetition time (TR): 2530 ms, echo time (TE): 2.98 ms, inversion time (TI): 1100 ms, FA:7°, field of view (FOV): 256 mm × 224 mm, matrix: 256 × 224, slice thickness: 1 mm, isotropic voxel size: 1 mm × 1 mm × 1 mm, number of slices:192, bandwidth:240 Hz/Px, and acquisition time: 5min58sec. The structural image data was preprocessed using the DPABISurf toolbox. We then compared the clinical characteristics between PD and healthy subjects. Furthermore, we analyzed the correlation between brain surface area and behavioral data. The Mini-Mental State Examination (MMSE) was used for behavioral and cognitive assessments. Gait can be modeled as a combination of five domains: pace, rhythm, variability, asymmetry and postural control. For all PD and control participants, objective gait assessments were assessed using a 20-foot-long computerized Zeno Walkway system (Proto Kinetics, Havertown, PA, USA) at a self-selected pace (SSP) and fast pace. PD participants were assessed with the following tests to characterize disease status and gait impairment: full MDS-UPDRS, Berg Balance Scale (BBS), Dynamic Gait Index, New Freezing of Gait Questionnaire (nFOGQ), and the Timed-Up and Go (TUG). These tests have been shown to be valid tools in PD assessment.Results
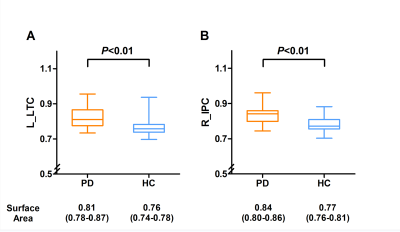
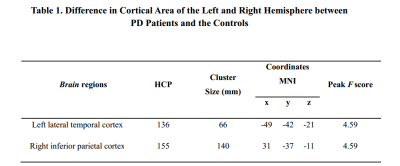
Surface Area of left LTC and right IPC is enlarged in the brain of PD subjectsSurface area morphometry results showed that the surface areas of the left lateral temporal cortex (LTC) and the right inferior parietal cortex (IPC) were significantly larger in the PD subjects compared to the healthy subjects (P<0.05 corrected by Monte Carlo simulation) (Figure 1, Table 1). The differences remained significantly larger after adjusting for age (P=0.0024) and gender (P=0.0083) (Figure 2).
Correlation Analysis shows association between increased brain surface area and gait in PD subjects
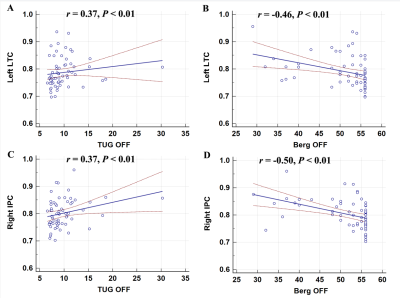
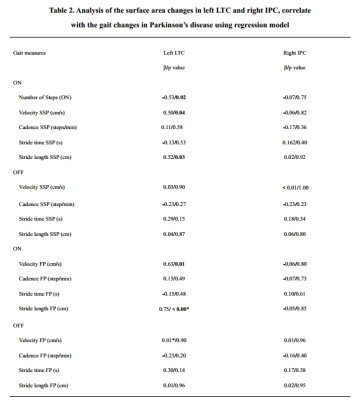
The surface area of the right IPC showed positive correlation with TUG-off test scores, but showed negative correlation with the Berg Balance Scale off (BBS-off) score (all P<0.01; Figure 3). Standardized linear regression analysis results showed that the surface area measures of the left LTC correlated with the objective gait parameters based on standardized linear regression analysis. In the ON medication state, larger left LTC surface area values showed negative correlation with the number of steps (P=0.02) and positive correlation with stride velocity self-selected pace (SSP) (P=0.04), stride length SSP (P =0.03), stride velocity fast pace (FP) (P=0.05), and stride length FP (P=0.002). The surface area in the right IPC did not show any significant correlation with quantitative gait parameters (Table 2).
Discussion
Kostic et al., reported that cortical surface area was significantly larger in PD patients compared to the healthy subjects [5]. Jubault et al., reported that the surface area of the brain cortex was related to the degree of local folding [6]. Cortical folding is universally related to surface area and thickness [7]. Moreover, greater tension or contraction of the white matter fibers increases the cortical surface area [8]. Our findings are consistent with previous studies.LTC was seen to be associated with the velocity, stride length and number of steps of the gait in the ON state, but there was no correlation in the OFF state, suggesting this correlation is dopamine-independent.Conclusions
Our findings suggest that PD is associated with a larger surface area alteration in the LTC and IPC. PD is associated with a characteristic pattern of cortical change in the left LTC and right IPC using surface-based analysis through both dopamine dependent and independent pathways. Such changes and their association gait impairment suggest a regional network failure. Overall, surface-based analysis might provide a useful tool for assessing PD for specific motor and non-motor functions, such as gait and cognition. Potentially, in conjunction with behavioral measures, the regional pattern of cortical change could serve as marker in monitoring disease progression.Acknowledgements
We especially thank Beijing Friendship Hospital, Capital Medical University, for the support with our research. We also thank all the study participants for their time and support.References
1. Michely, J., Volz, L.J., Barbe, M.T., Hoffstaedter, F., Viswanathan, S., Timmermann, L., et al. (2015). Dopaminergic modulation of motor network dynamics in Parkinson's disease. Brain. 138(Pt 3), 664-678.
2. Delgado-Alvarado, M., Gago, B., Navalpotro-Gomez, I., Jimenez-Urbieta, H., and Rodriguez-Oroz, M.C. (2016). Biomarkers for dementia and mild cognitive impairment in Parkinson's disease. Mov Disord. 31(6): 861-881.
3. Aarsland, D., Creese, B., Politis, M., Chaudhuri, K.R., Ffytche, D.H., Weintraub, D., et al. (2017). Cognitive decline in Parkinson disease. Nat Rev Neurol. 13(4): 217-231.
4. Camicioli, R., Oken, B.S., Sexton, G., Kaye, J.A., and Nutt, J.G. (1998). Verbal fluency task affects gait in Parkinson's disease with motor freezing. J Geriatr Psychiatry Neurol. 11(4): 181-185.
5. Kostic, V.S., Agosta, F., Pievani, M., Stefanova, E., Jecmenica-Lukic, M., Scarale, A., et al. (2012). Pattern of brain tissue loss associated with freezing of gait in Parkinson disease. Neurology. 78(6): 409-416.
6. Jubault, T., Gagnon, J.F., Karama, S., Ptito, A., Lafontaine, A.L., Evans, A.C., et al. (2011). Patterns of cortical thickness and surface area in early Parkinson's disease. Neuroimage. 55(2): 462-467.
7. Mota, B., and Herculano-Houzel, S. (2015). BRAIN STRUCTURE. Cortical folding scales universally with surface area and thickness, not number of neurons. Science. 349(6243), 74-77.
8. Van Essen, D.C. (1997). A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 385 (6614): 313-318.
Figures