2267
Brain correlates of voice disorders in early Parkinson’s disease1Centre de NeuroImagerie de Recherche – CENIR, Institut du Cerveau – ICM, Paris, France, 2Team “Movement Investigations and Therapeutics”(MOV'IT), Institut du Cerveau – ICM, Paris, France, 3Sorbonne University, Inserm, CNRS, Paris Brain Institute - ICM, Paris, France, 4Department of Neuroradiology, AP-HP, Pitié-Salpêtrière Hospital, Paris, France, 5Clinical Investigation Center for Neurosciences, Pitié-Salpêtrière Hospital, INSERM, Paris, France, 6Department of Neurology, AP-HP, Pitié-Salpêtrière Hospital, Paris, France, 7AP-HP, Pitié-Salpêtrière Hospital, Paris, France, 8PERFORM Centre, Electrical & Computer Engineering Department, Concordia University, Montreal, QC, Canada, 9Laboratoire SAMOVAR, Télécom SudParis, Institut Polytechnique de Paris, Paris, France, 10Sleep disorders unit, AP-HP, Pitié-Salpêtrière Hospital, Paris, France, 11Department of Nuclear Medicine, AP-HP, Pitié-Salpêtrière Hospital, Paris, France
Synopsis
We studied the neural mechanisms underlying Parkinson’s disease (PD) speech impairments. We assessed the relationships between the speech impairment in PD and changes in the nigrostriatal system (DAT imaging and neuromelanin-sensitive MRI) and in resting state fMRI functional connectivity within cortical language networks. Patients presented speech alterations depending on gender. Dopaminergic loss in the striatum as well as functional connectivity between the basal ganglia and the cortex were related to PD speech impairments in men, whereas in women only functional connectivity seemed to play a role in PD speech deterioration.
Introduction
Speech disorders, commonly described under the name of hypokinetic dysarthria1, are amongst the first symptoms to appear in Parkinson's disease (PD). They include a prosody reduction (monopitch and monoloudness), phonation irregularities, abnormal pauses, and altered articulation and rhythmic abilities. Although many studies have characterized PD speech impairments, their underlying pathophysiology is still partially understood, especially at the early stage of the disease. In addition, most of the studies were performed on small databases and did not analyze men and women separately, despite several sex differences in PD phenotypic expression, brain structure and dopaminergic changes2–4. In our study, we characterized the differences within the language networks, between early PD subjects and healthy controls (HC) and the influence of gender and we studied the relationship between quantitative speech impairments and multimodal imaging, in order to better understand the pathophysiology of hypokinetic dysarthria specific to each gender.Methods
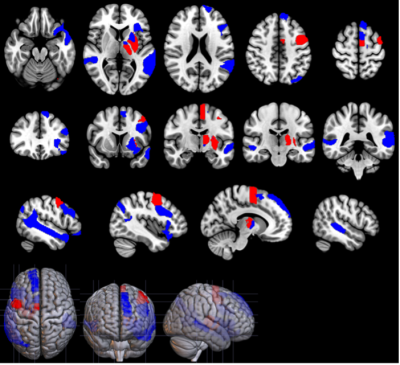
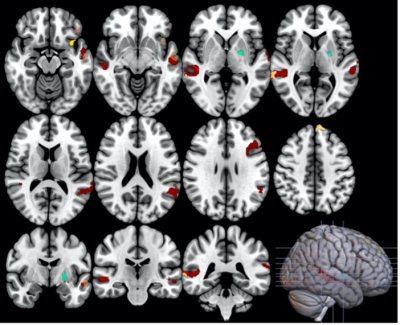
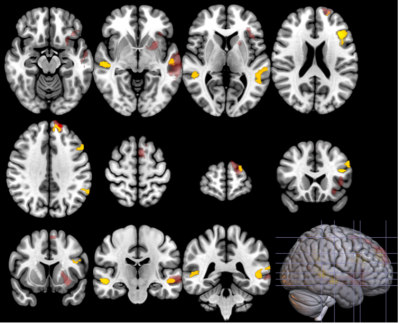
Data acquisition The database included 148 right-handed French speakers. 104 were recently diagnosed with PD (68M/36F) and 44 were HC (23M/21F). PD subjects had a disease duration of 2.6 ± 1.4 years, a Hoehn & Yahr stage of 2.0 ± 0.1 , and were pharmacologically treated. The participants performed different speech tasks, composed of sustained vowels, free speech, fast syllable repetitions, slow syllable repetitions following a 1 Hz given pace, short sentences repetition, and text, dialog and short sentences readings. They also completed multimodal imaging, including dopamine transporter imaging (DAT), MRI neuromelanin sensitive imaging and resting state functional MRI (rs-fMRI). Feature extraction Voice: Speech features related to prosody (standard deviation of pitch (SD log F0)), phonation (average score of jitter, shimmer and Noise-to-Harmonics ratio), speech fluency (median pause duration) and rhythmic abilities (Relative Standard Deviation (RSD) and average difference with the example) were extracted. To quantify the articulatory impairments, we used the scores resulting from gender specific classifiers based on Mel-frequency cepstral coefficients (MFCC): an MFCC-Gaussian Mixture Model for men and an x-vectors classifier for women5. DAT: Specific Binding Ratios (SBR) were obtained from DAT images in the sensorimotor and associative territories of the putamen and caudate nucleus, segmented using the YeB atlas6. Neuromelanin: The signal-to-noise ratios (SNR) of neuromelanin-sensitive MRI were calculated in sensorimotor and associative subregions of the Substantia Nigra (SN), relative to a background region of interest7. rs-fMRI: Functional connectivity was analyzed between basal-ganglia seeds (subregions used in the DAT and NM analyses) and two distinct language network masks (executive and associative) represented in Figure 1. Statistical analyses Group analyses consisted in univariate two-way ANOVAs with an interaction term, to assess the effects due to PD and gender and due to the interaction between both. In PD patients, partial correlation analyses were performed between the discriminant voice features and neuroimaging data. Men and women were considered separately to study the specificity of PD voice impairments neural correlates depending on the sex.Results
Group differences PD patients had a more monotonous voice, longer median pauses durations, difficulty maintaining a steady rhythm and a poorer articulation score than controls with an interaction effect (greater changes in PD men than women). PD patients had reduced SBR in both the left and right associative and sensorimotor territories of the putamen and the caudate nucleus. SBR was higher in women in all subregions. PD subjects had lower SN neuromelanin SNR in the left and right sensorimotor territories. Functional connectivity between basal ganglia and the language network was reduced in PD patients (Figure 2), with interactions between disease and sex in the associative network (Figure 3). Correlation of speech features with neuroimaging DAT: SBR in putamen and caudate nucleus correlated with prosody in PD men, in all subregions with predominance in right sensorimotor territories. No correlations were found in women. Neuromelanin: There were no significant correlations with the speech features. rs-fMRI: Prosody and articulation correlated with functional connectivity in PD females. Speech fluency, rhythm and articulation correlated with connectivity in PD males.Discussion
Our DAT results support previous evidences regarding the laterality mainly associated with the prosody8, the higher SBR in women, and the correlation of the SBR in putamen and monopitch in PD men only9. The NM results are coherent with the later onset of neuromelanin reduction in SN compared to the dopaminergic loss in the striatum0. In female patients, we showed that the different speech impairments were more likely to be only related to cortical and subcortical basal ganglia pathway, as hypothesized Rusz et al.9Conclusion
In conclusion we showed that speech networks were affected differently by PD depending on the gender, and that the dopaminergic loss in the striatum as well as subcortico-cortical pathways were related to PD speech impairments in men, whereas in women only the basal ganglia - cortical functional connectivity seemed to play a role in the speech deterioration.Acknowledgements
No acknowledgement found.References
1. Darley Frederic L., Aronson Arnold E., Brown Joe R. Differential Diagnostic Patterns of Dysarthria. Journal of Speech and Hearing Research. 1969;12:246–269.
2. Iwaki H, Blauwendraat C, Leonard HL, et al. Differences in the Presentation and Progression of Parkinson’s Disease by Sex. Mov Disord. 2021;36:106–117.
3. Tremblay C, Abbasi N, Zeighami Y, et al. Sex effects on brain structure in de novo Parkinson’s disease: a multimodal neuroimaging study. Brain : a journal of neurology. Epub 2020 Sep 27.
4. Kaasinen V, Joutsa J, Noponen T, Johansson J, Seppänen M. Effects of aging and gender on striatal and extrastriatal [123I]FP-CIT binding in Parkinson’s disease. Neurobiol Aging. 2015;36:1757–1763.
5. Jeancolas L, Petrovska-Delacrétaz D, Mangone G, et al. X-Vectors: New Quantitative Biomarkers for Early Parkinson’s Disease Detection From Speech. Front Neuroinform [online serial]. Frontiers; 2021;15. Accessed at: https://www.frontiersin.org/article/10.3389/fninf.2021.578369. Accessed February 19, 2021.
6. Bardinet E, Bhattacharjee M, Dormont D, et al. A three-dimensional histological atlas of the human basal ganglia. II. Atlas deformation strategy and evaluation in deep brain stimulation for Parkinson disease. Journal of Neurosurgery. 2009;110:208–219.
7. Biondetti E, Gaurav R, Yahia-Cherif L, et al. Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson’s disease. Brain : a journal of neurology. 2020;143.
8. Lindell AK. In Your Right Mind: Right Hemisphere Contributions to Language Processing and Production. Neuropsychol Rev. 2006;16:131–148.
9. Rusz J, Tykalová T, Novotný M, Zogala D, Růžička E, Dušek P. Automated speech analysis in early untreated Parkinson’s disease: relation to gender and dopaminergic transporter imaging. European Journal of Neurology [online serial]. 2021;n/a. Accessed at: http://onlinelibrary.wiley.com/doi/abs/10.1111/ene.15099. Accessed September 14, 2021.
10. Biondetti E, Santin MD, Valabrègue R, et al. The spatiotemporal changes in dopamine, neuromelanin and iron characterizing Parkinson’s disease. Brain [online serial]. Epub 2021 May 12. Accessed at: https://doi.org/10.1093/brain/awab191. Accessed September 9, 2021.
Figures