2254
Constant-Tissue Model for Comparison of Supine and Prone Breast Coil SNR1Department of Radiology, Stanford University, Stanford, CA, United States, 2Department of Bioengineering, Stanford University, Stanford, CA, United States, 3GE Healthcare, Aurora, OH, United States
Synopsis
Recent work has developed supine breast imaging for improved correlation with surgery and comfort but will likely require breast-specific coils to maximize the SNR. Comparing prone and supine SNR can be difficult due to substantial deformation between the positions and heterogeneity of the patient population. We measured SNR in a constant-tissue model to reduce dependence on tissue heterogeneity and provide an overall SNR across the volume of interest. We compared SNR of a novel 60-channel supine coil and a standard prone coil. In the cosntant-tissue images, the supine 60-channel coil increased SNR by 33%, 78% and 80% across 3 volunteers.
Introduction
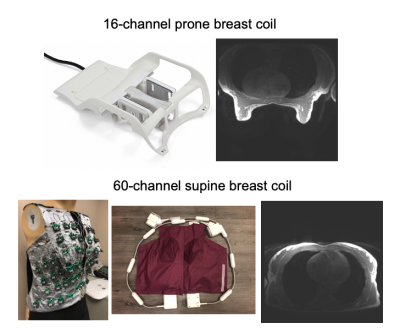
Breast MRI is typically acquired in the prone position to mitigate respiration. However, co-localization with biopsies, surgery, and other imaging is challenging. Additionally, prone breast coils are uncomfortable and large, limiting both the time a patient can remain still and the remaining bore space. Therefore, recent efforts have developed supine breast imaging methods1-5 and breast-specific coils to improve SNR and parallel imaging6 and mitigate respiratory motion.Generalizable approaches to quantifying SNR in breast imaging are difficult due to a wide range of breast size, shape, and composition of fibroglandular and adipose tissue, which influence SNR. Even within the same participant, comparing prone and supine coils is challenging because the breast shape and composition varies significantly between the positions [Fig1]. Direct comparison of SNR in specific features requires image registration7, which is nontrivial due to the substantial 3D deformation. The purpose of this work was to develop and apply a simple method to quantitatively compare prone and supine breast SNR distribution using a constant-tissue model and standard SNR measurement approach.
Methods
AcquisitionWe acquired fully-sampled 3D T1-weighted SPGR images (TR=3.67 ms, TE=2.1 ms, flip angle=12°) on a 3T Premier (GE Healthcare) using the 16-channel Sentinelle prone breast coil and the 60-channel breast coil prototype6. In a phantom experiment, two deformable uniform-signal saline bags represented the breasts. Data were also acquired in three healthy volunteers in both positions with slices and FOV chosen to fit the breast with of 2.2 mm isotropic resolution.
Method
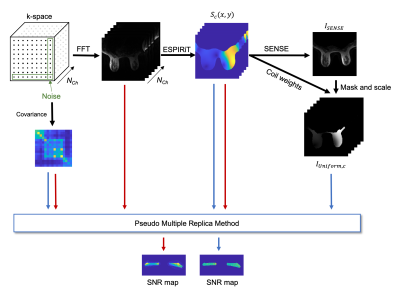
Figure 2 shows the methods pipeline, which uses a standard SENSE reconstruction and breast segmentation to generate “constant-tissue” source images for tissue-independent SNR measurements.
Coil sensitivity maps (Sc, for channel, c, from 1 to Nch) were estimated from fully-sampled k-space with ESPIRiT8. Channels were combined using SENSE R=1, and the resulting image (ISENSE(x,y,z)) was used to manually mask the breast region on a single, center slice (in vivo) or automatically mask the volume (phantom), mask(x,y,z). The mask was used to create a “constant-tissue” image where the magnitude was defined as the average signal over the mask, weighted voxel-wise by the coil sensitivities: $$I_{uniform,c}(x,y,z)=mask(x,y,z)×S_c (x,y,z)×\frac{1}{N_{mask}}∑_{mask}^{N_{mask}}I_{SENSE}(x,y,z)$$ The SNR was calculated for both the original imaging data and the constant-tissue image using the pseudo multiple replica method9 with 50 iterations, where the noise covariance was defined based on the edges of k-space. SNR was evaluated over the masked slice or volume.
Results
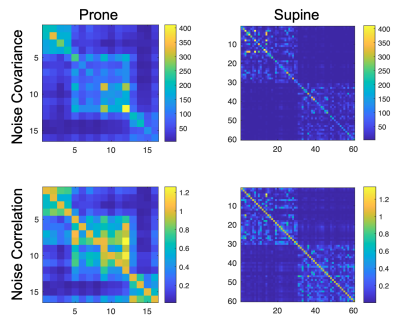
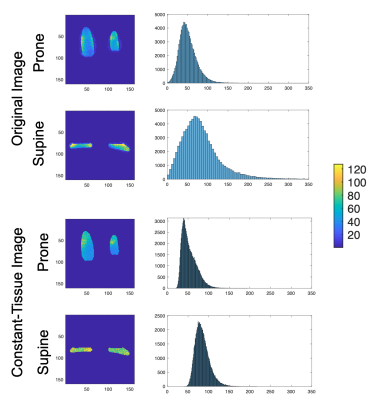
The noise covariance and correlation are shown in Figure 3. The prototype 60-channel coil has reasonably low correlation and similar noise levels across channels compared to the prone coil.SNR of the constant-tissue image was compared to that of the original image in a uniform-signal phantom in Figure 4. SNR of the original image is dependent on the varying coil intensities but was more uniform across the constant-tissue image where spatial variation was caused by the coil sensitivities. The supine coil showed higher overall SNR across the volume, as demonstrated in the histograms. As expected, the prone/supine SNR difference was somewhat consistent across the original and constant-tissue images.
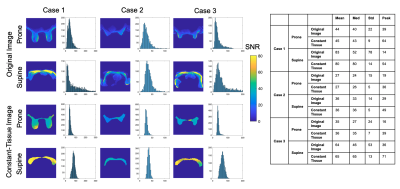
Figure 5 shows SNR measured in a center slice in three in vivo examples. Overall SNR distributions were similar between the original and constant-tissue images. In the original images, the SNR varied with signal intensity and was inflated in the fatty tissue near the coil, whereas SNR had a narrower distribution across the slice in the constant-tissue images. Histogram statistics confirmed consistency between the original and constant-tissue images, reduced standard deviation using the constant-tissue image, and mean SNR 33, 78 and 80% higher in the supine coil than the prone coil.
Discussion
High SNR within the breast tissue is paramount for high resolution, diffusion imaging, acceleration... The SNR can depend on coil positioning and be difficult to measure across heterogenous populations. An alternative approach could measure the SNR within known lesions or predetermined features; however, the position of such features varies greatly across patients and would require many more scans to characterize the full volume SNR. We applied an efficient, novel approach to compare SNR between supine and prone breast imaging, which can be generalized to various anatomies and extended to include g-factor estimation. Based on these results, we expect that the supine coil will provide lower g-factors, which, paired with the higher unaccelerated SNR, will greatly improve the acceleration ability.Because the method relies on an accurate measurement of the average signal in a volume of interest, we used manually drawn masks of a single slices to minimize bias. Future work will extend to a full volume with accurate automatic masking of the breast tissue.
While the 60-channel breast coil is referred to, herein, as the supine coil, it can be used prone with additional support; further prone assessment is needed.
Conclusion
We measured SNR in a digital constant-tissue image to compare different coil geometries in a way that represents the overall signal intensity but is independent of the spatially varying tissue composition.The supine coil increased SNR by 33, 78 and 80% across three in vivo examples, as expected due to the proximity to the breasts and large number of small surface elements consistent with the flatter breast shape in the supine position.
Acknowledgements
We gratefully acknowledge the research support of GE Healthcare and the following funding sources: NIH/NIBIB R01 EB009055 and NIH/NCI R01 CA249893.References
1. Fausto A, Fanizzi A, Volterrani L, et al. Feasibility, Image Quality and Clinical Evaluation of Contrast-Enhanced Breast MRI Performed in a Supine Position Compared to the Standard Prone Position. Cancers (Basel). 2020;12.
2. Gombos EC, Jayender J, Richman DM, et al. Intraoperative Supine Breast MR Imaging to Quantify Tumor Deformation and Detection of Residual Breast Cancer: Preliminary Results. Radiology. 2016;281:720-729.
3. Joukainen S, Masarwah A, Kononen M, et al. Feasibility of mapping breast cancer with supine breast MRI in patients scheduled for oncoplastic surgery. Eur Radiol. 2019;29:1435-1443.
4. Mallory MA, Sagara Y, Aydogan F, et al. Feasibility of Intraoperative Breast MRI and the Role of Prone Versus Supine Positioning in Surgical Planning for Breast-Conserving Surgery. Breast J. 2017;23:713-717.
5. Wu ZY, Alzuhair A, Kim H, et al. Magnetic resonance imaging based 3-dimensional printed breast surgical guide for breast-conserving surgery in ductal carcinoma in situ: a clinical trial. Sci Rep. 2020;10:18534.
6. Vincent J. Proc. Intl. Soc. Mag. Reson. Med. 29 (2021) 1591
7. Nnewihe AN, Daniel BL, Qiu D, Lipson J, Rosenberg J, Ikeda D, Kim SH, Kao J, Hargreaves BA. A Novel Method for Coil Array Synthesis and Application to Breast MRI. 20th Scientific Meeting of the ISMRM, Melbourne, Australia, 2012; p. 2535.
8. BART Toolbox for Computational Magnetic Resonance Imaging, DOI: 10.5281/zenodo.592960
9. Robson, P.M., et al. Magnetic resonance in medicine (2008). 60(4), 895–907. https://doi.org/10.1002/mrm.21728
Figures