2249
Characterization of tumor mechanical properties in glioma patients using 3D phase-gradient inversion in multifrequency MR elastography1Department of Radiology, Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany, 2Faculty of Physics and Earth Sciences, Peter Debye Institute, Leipzig University, Leipzig, Germany, 3Department of Neuropathology, Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany, 4Institute of Medical Informatics, Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany, 5Institute of Neuroradiology, Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany, 6Department of Neurosurgery, Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany, 7Department of Neurosurgery, University Hospital Frankfurt, Frankfurt am Main, Germany
Synopsis
Clinical MRI is an important method for the delineation and characterization of gliomas for resection planning and therapy monitoring in neurosurgery. Here, we used a 3D curl- and phase-gradient based inversion algorithm in multifrequency magnetic resonance elastography (MRE) to generate maps of the mechanical tumor properties, wave speed and wave damping. Overall, our method improved the spatial resolution of MRE maps in glioma patients and allowed precise measurement of tumor mechanical properties. In the future, the method could help with characterization, surgical planning and treatment monitoring of neuro tumors.
Introduction
Gliomas are the most common primary brain tumors with a very poor survival rate. Especially patients with a grade IV astrocytoma (glioblastoma), which makes up 75% of all glioma cases, have a 5-year survival rate of only 7%.1 Clinical MRI plays a crucial role in detection, delineation and characterization of gliomas for resection planning and therapy monitoring in neurosurgery.2 Conventional imaging biomarkers are limited in their sensitivity to depict tumor borders, histological subtype and mutation state.2 Thus, conventional imaging cannot always provide information about tumor stage and aggressiveness. Magnetic resonance elastography (MRE) quantifies the mechanical properties of soft tissue and can provide additional information for clinical tumor grading and surgery planning. So far, only limited MRE data has been reported in glioma.3,4 The aim of this study was to use multifrequency MRE combined with a 3D phase-gradient based inversion algorithm to acquire high resolution maps of stiffness and wave penetration in glioma patients.Methods
The study protocol is in accordance with the Declaration of Helsinki and was approved by our institutional ethics review board. All patients gave written informed consent. Overall, 23 patients with glioma were analyzed, from whom 3, 7 and 13 had a grade II, grade III and grade IV glioma, respectively. All patients underwent presurgical clinical contrast enhanced (CE) MRI, diffusion weighted MRI and multifrequency MRE. MRE was performed on a 3-Tesla MRI scanner (Magnetom Lumina, Siemens Healthineers) using a single-shot, spin-echo, EPI sequence with externally induced vibrations of 20, 25, 30 and 40 Hz. Eight phase steps equally spaced over a full vibration cycle were acquired with 3D flow-compensated motion encoding gradients.5 Up to 36 images slices with a field of view (FOV) of 201×201 mm² and a resolution of 1.6×1.6×2 mm³ were acquired. Total scan time for a full multifrequency MRE data set was approximately 7 min. After 2D motion correction, MRE phase images were unwrapped, Fourier transformed and corrected for phase offsets between slices.6,7 3D curl fields were calculated and decomposed into 20 3D wave propagation directions.7 MRE data were then smoothed with a first order low-pass Butterworth filter with a threshold of 200 m-1. Maps of shear wave speed (SWS) and penetration rate (PR) were generated based on a 3D phase-gradient inversion. Contrast enhanced (CE) MPRAGE, T2w images and apparent diffusion coefficient (ADC) from diffusion weighted images (DWI) were registered to MRE data using ITKsnap.8 Volume of interest (VOI) of gliomas were manually drawn based on CE MPRAGE, T2w, ADC and MRE magnitude, excluding cystic regions. For contralateral normal appearing white matter (NAWM), the glioma VOIs were flipped and adjusted for sulci. Glioma and NAWM values of SWS, PR and ADC were calculated. After biopsy or surgical resection, histological tumor stains of hematoxylin and eosin (HE), elastica van gieson (EvG), reticulin, CD31, p53 were conducted.Results
Figure 1 shows a representative slice of CE MPRAGE, curl wave fields, MRE maps and ADC of a grade II astrocytoma in the left frontal lobe. The low-grade glioma appears as a slightly softer mass with a reduced penetrations rate and increased ADC compared to the NAWM. Figure 2 shows representative elastograms and ADC of a grade III oligodendroglioma and a grade IV astrocytoma.Mean SWS was 0.91±0.08m/s, 1.14±0.16 m/s, and 0.88±0.15 m/s, for grade II, III and IV gliomas, respectively. Mean PR was 0.48±0.07 m/s, 0.59±0.09 m/s, and 0.48±0.1 m/s, for grade II, III and IV gliomas, respectively. Mean ADC was (1.59±0.28)×10-3 mm²/s for grade II, (1.27±0.03)×10-3 mm²/s for grade III, and (1.15±0.23)×10-3 mm²/s for grade IV gliomas. SWS was lower in gliomas than in NAWM (mean difference: -0.15±0.19 m/s, P<0.001), whereas ADC was increased (mean difference: (-0.4±0.3)×10-3 mm²/s, P<0.001). Figure 3 shows box plots of the mechanical parameters grouped by WHO grade.
Grade II and III glioma showed higher SWS values than glioblastoma (grade IV) (1.07±0.17 m/s vs. 0.88±0.15 m/s, P<0.05), while no difference was observed for ADC and PR (P=0.056 and P=0.074, respectively). Soft glioblastoma tended to be heterogeneous in terms of SWS. Figure 4 demonstrates a heterogenous, yet rather stiff glioblastoma that was described as firm mass by the neurosurgeon. Histopathological examination revealed high accumulation of elastin and reticulin in the ECM of this glioblastoma. Automatic and quantifiable analyses of histological stains are ongoing to reveal the association of tumor microenvironment with MRE parameters (Figure 5).
Discussion and Conclusion:
This study shows the feasibility of 3D curl- and phase-gradient inversion-based multifrequency MRE in glioma patients. Overall, the proposed method allows generation of high-resolution MRE maps of shear wave speed and penetration rate of the brain including glioma. We reproduced previous results, which indicate that gliomas are softer than NAWM.3 Although glioblastoma are heterogeneous in terms of morphology and viscoelastic properties, they are softer than grade II and III gliomas. To understand the micromechanical changes that determine the macroscopic mechanical properties and water diffusivity of glioma across different WHO grades, a quantitative histopathological analysis is needed and currently performed in our institution. The proposed multifrequency MRE protocol and inversion pipeline can easily be integrated into a standard neuroradiological workflow, thereby adding valuable information of tumor consistency and aggressiveness for surgical planning and therapy monitoring.Acknowledgements
Funding from the German Research Foundation (Sa901/17-2, GRK 2260 BIOQIC, SFB1340 Matrix in Vision) is gratefully acknowledged.References
1. Robert-Koch-Institut & Gesellschaft der epidemiologischen Krebsregister in Deutschland e, V. Krebs in Deutschland 2015/2016, (Berlin, 2019).
2. Weller, M., et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. The Lancet Oncology 18, e315-e329 (2017).
3. Pepin, K.M., et al. MR Elastography Analysis of Glioma Stiffness and IDH1-Mutation Status. AJNR Am J Neuroradiol 39, 31-36 (2018).
4. Bunevicius, A., Schregel, K., Sinkus, R., Golby, A. & Patz, S. REVIEW: MR elastography of brain tumors. NeuroImage: Clinical 25, 102109 (2020).
5. Hirsch, S., Braun, J. & Sack, I. Magnetic Resonance Elastography: Physical Background And Medical Applications, (Wiley-VCH, Weinheim, 2017).
6. Shahryari, M., et al. Reduction of breathing artifacts in multifrequency magnetic resonance elastography of the abdomen. Magnetic Resonance in Medicine 85(2020).
7. Herthum, H., et al. Magnetic resonance elastography of the in vivo human brain using multifrequency wavenumber analysis in 2D and 3D. In Proceedings of the 28th Annual Meeting of ISMRM. Virtual Conference. Abstract nr. 1023, (2021).
8. McCormick, M., Liu, X., Ibanez, L., Jomier, J. & Marion, C. ITK: enabling reproducible research and open science. Frontiers in Neuroinformatics 8(2014).
Figures
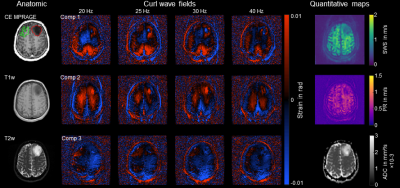
Figure 1: Contrast enhanced (CE) MPRAGE, curl wave fields, elastograms and ADC of a grade II astrocytoma. The glioma (red VOI) is discernible in the curl wave fields and elastograms based on mechanical properties and shows an increased ADC compared to NAWM (green VOI). SWS, shear wave speed in m/s; PR, penetration rate in m/s; ADC, apparent diffusion coefficient in mm²/s; VOI, volume of interest; NAWM, normal appearing white matter.
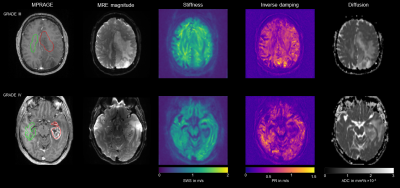
Figure 2: MRE magnitude, contrast enhanced MPRAGE, elastograms and ADC of a grade III oligodendroglioma (red VOI) and a grade IV astrocytoma. The oligodendrogliome shows an increased ADC, while mechanical properties do not seem to be much altered compared to NAWM (green VOI). The glioblastoma has a low SWS, low PR and a high ADC. SWS, shear wave speed in m/s; PR, penetration rate in m/s; ADC, apparent diffusion coefficient in mm²/s; NAWM, normal appearing white matter; VOI, volume of interest.
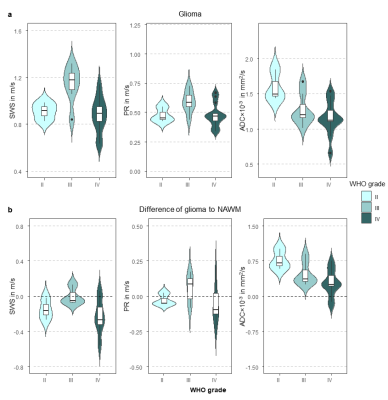
Figure 3: Box plot illustrating the mechanical properties and ADC of different glioma grades. a) Mechanical properties and ADC of gliomas. b) Mechanical properties and ADC of the difference between glioma and NAWM. SWS, shear wave speed in m/s; PR, penetration rate in m/s; ADC, apparent diffusion coefficient in mm²/s.
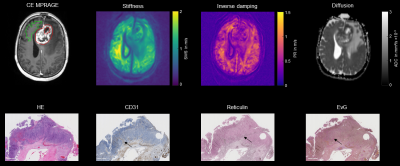
Figure 4: Shown are contrast enhanced (CE) MPRAGE, elastograms and histological stains of HE, elastin, reticulin and CD31 of a grade IV astrocytoma in the frontal lobe (red VOI). In MRE the glioblastoma is heterogenous yet stiff. In the histopathological stains, neoangiogenesis (CD31), unusual accumulation of reticulin and elastin (EvG) is observed (black arrows). SWS, shear wave speed in m/s; PR, penetration rate in m/s; ADC, apparent diffusion coefficient in mm²/s, VOI, volume of interest.
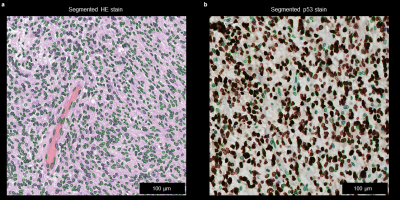
Figure 5: Automatic nucleus segmentation in HE and p53 stains of a glioblastoma (WHO-Grade IV astrocytoma). In HE (a) nucleus are segmented and quantified. In p53 stains apoptosis is quantified by segmentation of stained and unstained nuclei.