2247
Tumor-liver biomechanical interaction investigated by multifrequency MR elastography in patients with HCC
Jiahao Zhou1, Ruokun Li2, Yikun Wang2, Jing Guo3, Ingolf Sack3, and Fuhua Yan2
1radiology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China, 2Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China, 3Department of Radiology, Charité–Universitätsmedizin Berlin, Berlin, Germany, Berlin, Germany
1radiology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China, 2Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China, 3Department of Radiology, Charité–Universitätsmedizin Berlin, Berlin, Germany, Berlin, Germany
Synopsis
Tumor-liver biomechanical interaction investigated by multifrequency MR elastography in patients with HCC
Synopsis
Hepatocellular carcinomas (HCCs) in noncirrhotic livers have a more favorable prognosis than HCCs that grow in cirrhotic livers. However, little is known why HCC aggressiveness is influenced by the properties of the surrounding tissue. Previous work indicated that HCCs favorably grow in stiff livers prompting the hypothesis that liver tumor aggressiveness is influenced by the mechanical properties of the surrounding tissue. Therefore, we here study the biomechanical properties of HCC and their hosting liver using tomoelastography, a multifrequency MR-elastography (MRE) technique. Our results show that HCCs in cirrhotic livers with poorer prognosis presented higher stiffness and increased fluidity, suggesting that the biomechanical properties of the hosting environment influences the aggressiveness the tumor.Introduction
HCCs commonly develop in liver cirrhosis by multistep hepatocarcinogenesis. However, approximately 20% of HCCs precede the occurrence of cirrhosis through distinct cytogenetic pathways1. Due to the lack of surveillance strategies, HCC in noncirrhotic liver usually manifest with a larger tumor burden and an advanced stage at the time of diagnosis. Nevertheless, these patients present with a more favorable prognosis. To date, little is known about the causes of the differential aggressiveness of HCCs growing in cirrhotic livers and HCCs growing in noncirrhotic settings. The interplay between the tumor entity and the Tumor Surrounding Environment (TSE) plays an important role during tumor progression2. This tumor-TSE interaction potentially changes the composition and the structure of the tissue and alters the biomechanical properties of the affected tissue. Tomoelastography, a multifrequency MR elastography (MRE) technique, can quantitatively map soft tissue viscoelasticity based on shear wave speed (c in m/s) and loss angle (φ in rad). Both parameters are used as surrogate markers of stiffness and viscosity or tissue fluidity, respectively. The present study aimed to investigate the tumor-liver biomechanical interaction in patients with HCC.Methods
This prospective study included 107 HCC patients (mean age, 59 years ± 12;90 men) who underwent preoperative MRI and tomoelastography. All participants were divided into two groups of patients with non-cirrhotic and cirrhotic livers based on histopathological analysis. The non-cirrhotic group included 51 patients with 52 lesions (mean age, 61 years ± 13;47 men) while the group of patients with cirrhosis included 56 patients with 61 lesions (mean age, 58 years ± 11;43 men). All tomoelastography experiments were performed on a clinical 1.5-Tesla MRI scanner (Magnetom Aera, Siemens, Erlangen, Germany) using four vibration frequencies (30Hz, 40Hz, 50Hz, 60Hz) and the multifrequency processing pipeline available at https://bioqic-apps.com[1] to generate full field-of-view maps of c and φ. Two radiologists independently evaluated the tomoelastography data. Interobserver agreement was assessed by the intraclass correlation coefficient (ICC). The biomechanical properties of HCC and liver parenchyma were compared between the non-cirrhotic and cirrhotic group using t-test. Pearson’s linear correlation coefficient was determined for tomoelastography parameters versus fibrosis grade as well as inflammation activity of the background liver. Pls check, the URL has charite in itDiscussion
Our study showed that biomechanical properties of the HCCs significantly depend on the viscoelasticity of the hosting liver. Cirrhotic livers fostered HCCs with stiffer, yet more fluid-like properties. This seemingly contradictory material’s behavior can be explained with the accumulation of matrix proteins and higher inflammation activity yielding, in cirrhotic livers, an increase in the both stiffness and viscoelastic dispersion slope or loss angle φ , respectively 3. In agreement to 3, we observed that the φ value of non-cirrhotic HCC-hosting liver was lower than π/4 (indicated predominantly solid properties) while the φ of cirrhotic liver was higher than π/4 (indicated predominantly fluid properties). This transition from solid to fluid along with soft to stiff indicates a significant increase in intrinsic mechanical friction, which likely forms a cancerous environment fostering HCCs with more aggressive properties. Furthermore, it is known that cancer cells act collectively. Unjammed cellular streams in HCC could influence the fluid properties of a tumor at the macroscopic level, indicating an increased metastatic potential 4. Probably for this reason, higher fluidity has been associated with higher aggressiveness of tumors 5,6. The higher fluidity that we observed in the HCCs of the cirrhotic liver is consistent with the clinical observation that HCC patients with liver cirrhosis show lower overall survival rates than those with non-cirrhotic livers. The more aggressive HCCs in the cirrhotic livers also displayed higher stiffness than the HCCs in the non-cirrhotic livers. This observation is in line with previous studies showing that higher tumor stiffness is associated with higher malignancy 5-7.Conclusion
Cirrhotic livers fostered HCCs that were stiffer and behaved more fluid-like. The biomechanical interaction between HCCs and their hosting liver might be an important predictive factor for tumor aggressiveness.Acknowledgements
No acknowledgement foundReferences
1 Desai, A., Sandhu, S., Lai, J. & Sandhu, D. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World journal of hepatology 11, 1-18, doi:10.4254/wjh.v11.i1.1 (2019). 2 Mierke, C. et al. The two faces of enhanced stroma: Stroma acts as a tumor promoter and a steric obstacle. NMR in biomedicine 31, e3831, doi:10.1002/nbm.3831 (2018). 3 Reiter, R. et al. Influence of fibrosis progression on the viscous properties of in vivo liver tissue elucidated by shear wave dispersion in multifrequency MR elastography. J Mech Behav Biomed Mater 121, 104645, doi:10.1016/j.jmbbm.2021.104645 (2021). 4 Friedl, P. & Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10, 445-457, doi:10.1038/nrm2720 (2009). 5 Li, M. et al. Tomoelastography Based on Multifrequency MR Elastography for Prostate Cancer Detection: Comparison with Multiparametric MRI. Radiology, 201852, doi:10.1148/radiol.2021201852 (2021). 6 Shahryari, M. et al. Tomoelastography Distinguishes Noninvasively between Benign and Malignant Liver Lesions. Cancer Res, doi:10.1158/0008-5472.CAN-19-2150 (2019). 7 Zhu, L. et al. Distinguishing pancreatic cancer and autoimmune pancreatitis with in vivo tomoelastography. Eur Radiol, doi:10.1007/s00330-020-07420-5 (2020).Figures
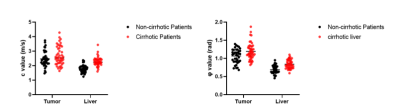
Figure 1: Comparison of the biomechanical properties of the HCC and the
HCC-hosting liver in patient with non-cirrhotic and
cirrhotic livers.
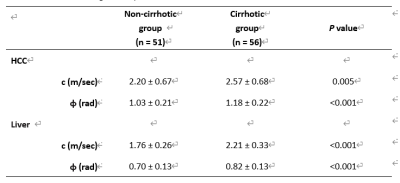
Table 1: Group mean values and the standard`
deviations of the biomechanical properties of the HCC and the HCC-hosting liver
in patient with non-cirrhotic and cirrhotic livers.
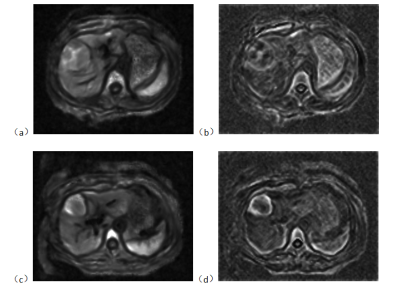
Figure 2: C-map (a) and φ-map (b) of a non-cirrhotic patient. C-map (c) and φ-map (d) of a cirrhotic patient.
DOI: https://doi.org/10.58530/2022/2247