2243
Quantitative server-based analysis of MR elastography inversion methods on a phantom and the in vivo human kidney1Department of Radiology, Charité - Universitätsmedizin Berlin, Berlin, Germany, 2Institute of Medical Informatics, Charité - Universitätsmedizin Berlin, Berlin, Germany, 3Department of Biomedical Engineering, The Ohio State University, Columbus, OH, United States, 4Department of Radiology, The Ohio State University, Columbus, OH, United States
Synopsis
Magnetic resonance elastography (MRE) depicts the viscoelastic properties of soft tissues for diagnosis of various diseases such as tumors or fibrosis. However, different MRE inversion methods yield different results. This lack of comparability of different MRE inversion results hinders the dissemination of advanced reconstruction methods. Therefore, we here introduce an extensible, open-access web platform that offers multiple inversion techniques for multifrequency, three-dimensional MRE to promote comparison of values. We demonstrate the utility of the platform in phantom data and in vivo free-breathing multifrequency MRE data of the kidneys of healthy volunteers.
Introduction
Magnetic resonance elastography (MRE)1 is an established technique for staging liver fibrosis2 and is currently being developed for other diagnostic applications, including brain disease3 and tumors4. However, different MRE methods measure different values in the same tissue, leading to inconsistent thresholds for viscoelasticity-based detection of disease.We hypothesize that comparison of values, assessment of data quality, consistency of MRE results, and, ultimately, standardization of thresholds for quantification of disease can be critically advanced by providing an open platform where all types of MRE data can be processed using various established inversion methods. Therefore, we here introduce such a platform, implemented as an open access web application. We demonstrate the utility of the proposed platform in a phantom and in a set of prospectively acquired multifrequency MRE data of the healthy human kidney.
Methods
Phantom DataFor validation, repository data of an agar-based Wirogel (Bego Inc., Bremen, Germany) phantom9 with four cylindrical inclusions was used.
Kidney Data
The study was approved by our ethics committee. 23 healthy volunteers (10 women, mean age 35±11 years, age range: 21 to 53 years) were studied by multifrequency MRE in a 1.5-Tesla MRI scanner (Magnetom Sonata, Siemens, Erlangen)5, after written informed consent was obtained. Multifrequency vibrations of 40, 50, 60, and 70-Hz frequencies were consecutively induced by pressurized-air driven actuators. Full wavefields were acquired in eleven contiguous paracoronal slices using a single-shot, spin-echo planar imaging sequence with a 256×256mm² field-of-view and 2.46×2.46×2.5mm³ voxels.
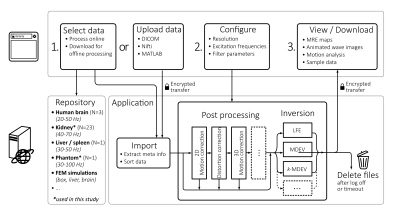
Server Architecture
The MRE processing platform (https://bioqic-apps.charite.de/) was implemented as a Java web application. The Java application handles downloads and uploads of imaging data and runs compiled MATLAB scripts for data processing. Communication with web server was encrypted using HTTPS and data is automatically deleted after logging off or time-out. The pipelines can be parameterized by default or optimized data-specific settings. The server architecture is presented in figure 1.
Inversion methods: MDEV, k-MDEV and LFE
MDEV inversion is an approach to Helmholtz (‘direct’) inversion, providing the magnitude of the shear modulus (|G*| in kPa) and ϕ, the loss angle6,7. k-MDEV analyzes the phase-gradient of plane waves by 2D-first-order derivatives, waves are decomposed into plane-waves using directional filters8, from which shear-wave speed (SWS in m/s, related to stiffness) and wave penetration rate (PR in m/s, related to inverse wave attenuation) are obtained by inversion.9 LFE exploits six pairs of lognormal filters and |G*| is obtained from the ratio of shear wave fields filtered with a pair of lognormal filters10.
Data were processed after 2D- and 3D-motion correction16,11. Stiffness was converted to SWS by sqrt(|G*|/1kg/L) assuming unit mass density. Regions of interest (ROIs) of the kidney were manually drawn for the medulla, inner- and outer cortex based on MRE magnitude images. Group was performed using one-way ANOVA for repeated measures with Bonferroni correction and Tukey's test for post-hoc analysis.
Results
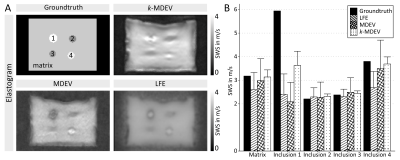
Phantom analysisFigure 2 shows ground-truth and results for phantom data using LFE, MDEV and k-MDEV. While all methods agreed with ground-truth for matrix and softer inclusions, there was larger disparity for stiff inclusions. Only k-MDEV detected that inclusion 1 was stiffer than the matrix, however MDEV and k-MDEV correctly detected inclusion 4.
In vivo kidney analysis
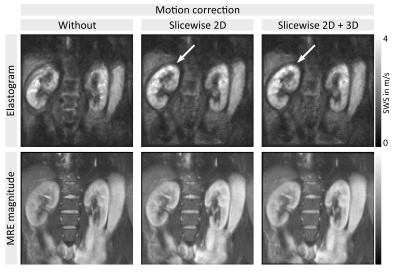
SWS values of the full parenchyma were uninfluenced by motion correction, corroborating previous findings11, however given the clear improvement in image sharpness (figure 3), 2D-motion correction was added as a preprocessing module to all methods. Representative slices of kidney SWS obtained by LFE, MDEV and k-MDEV are shown in figure 4. k-MDEV SWS values were higher than MDEV and LFE values (full-kidney SWS: 2.71±0.19m/s [k-MDEV], 2.14±0.16m/s [MDEV], 2.12±0.15m/s [LFE], each p<0.001). k-MDEV visualized renal substructures in greater detail than the other two methods, however consistently, all methods showed the inner renal cortex to be stiffer than medulla and outer cortex without differences between right and left kidney (all p>0.05, figure 5).
Discussion
We here presented an open-access platform for multifrequency, three-dimensional MRE data offering comparative processing using multiple inversion methods. Beyond wave inversion with standardized pipelines of LFE, MDEV and k-MDEV, the platform incorporates modular preprocessing blocks such as 2D- and 3D-motion correction16,11 and distortion correction12 as well as an repository of MRE data for download and online processing to which the community has contributed13,14.Beyond presenting novel MRE processing tools, we here presented the first comparative analysis of renal stiffness encompassing multiple inversion pipelines with motion-corrected multifrequency wave data. Our results are consistent with renal stiffness measured by tomoelastography (SWS=2.34±0.15m/s8, 2.40±0.17m/s15, based on k-MDEV inversion) and single-frequency MRE (60Hz, 1.82±0.54m/s based on LFE10). Tomoelastography consistently showed that the inner cortex was stiffer than medulla and outer cortex (2.92±0.25m/s, 2.41±0.13m/s, 2.16±0.18m/s)15, previous work based on LFE did not resolve inner and outer cortex10.
Conclusion
We addressed the well-known lack of comparability of MRE results by introducing an expandable, easy-to-use, open-access platform that offers inversion techniques for multifrequency, 3D-MRE including LFE, MDEV and k-MDEV inversion. We demonstrated the utility of the platform in phantom data and in kidneys of healthy subjects. The platform might foster the development of quantitative imaging biomarkers related to tissue viscoelasticity by facilitating analysis of effects of filter thresholds, spatial resolution, motion and frequency dispersion on MRE results.Acknowledgements
Support of the German Research Foundation (GRK2260 BIOQIC, SFB1340, project number 467843609) is gratefully acknowledged.References
- Muthupillai, R. & Ehman, R. L. Magnetic resonance elastography. Nature Med. 2, 601-603 (1996).
- Kennedy, P. et al. Quantitative Elastography Methods in Liver Disease: Current Evidence and Future Directions. Radiology 286, 738-763, doi:10.1148/radiol.2018170601 (2018).
- Hiscox, L. V. et al. Magnetic resonance elastography (MRE) of the human brain: technique, findings and clinical applications. Phys Med Biol 61, R401-R437 (2016).
- Garteiser, P. et al. MR elastography of liver tumours: value of viscoelastic properties for tumour characterisation. Eur Radiol 22, 2169-2177 (2012).
- Lang, S. T. et al. Multiparametric Quantitative MRI for the Detection of IgA Nephropathy Using Tomoelastography, DWI, and BOLD Imaging. Invest Radiol 54, 669-674, doi:10.1097/RLI.0000000000000585 (2019).
- Papazoglou, S., Hirsch, S., Braun, J. & Sack, I. Multifrequency inversion in magnetic resonance elastography. Phys Med Biol 57, 2329-2346 (2012).
- Streitberger, K.-J. et al. High-resolution mechanical imaging of glioblastoma by multifrequency magnetic resonance elastography. PLoS One 9, e110588 (2014).
- Manduca, A., Lake, D. S., Kruse, S. A. & Ehman, R. L. Spatio-temporal directional filtering for improved inversion of MR elastography images. Med Image Anal 7, 465-473 (2003).
- Tzschatzsch, H. et al. Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves. Medical Image Analysis 30, 1-10, doi:10.1016/j.media.2016.01.001 (2016).
- Gandhi, D. et al. Magnetic resonance elastography-derived stiffness of the kidneys and its correlation with water perfusion. Nmr in Biomedicine 33, doi:10.1002/nbm.4237 (2020).
- Shahryari, M. et al. Reduction of breathing artifacts in multifrequency magnetic resonance elastography of the abdomen. Magn Reson Med, doi:10.1002/mrm.28558 (2020).
- Fehlner, A. et al. Increasing the spatial resolution and sensitivity of magnetic resonance elastography by correcting for subject motion and susceptibility-induced image distortions. Journal of Magnetic Resonance Imaging 46, 134-141, doi:10.1002/jmri.25516 (2017).
- Barnhill, E. et al. Fast Robust Dejitter and Interslice Discontinuity Removal in MRI Phase Acquisitions: Application to Magnetic Resonance Elastography. IEEE Trans Med Imaging 38, 1578-1587, doi:10.1109/TMI.2019.2893369 (2019).
- Ariyurek, C., Tasdelen, B., Ider, Y. Z. & Atalar, E. SNR Weighting for Shear Wave Speed Reconstruction in Tomoelastography. NMR Biomed, e4413, doi:10.1002/nbm.4413 (2020).
- Marticorena Garcia, S. R. et al. Tomoelastography Paired With T2* Magnetic Resonance Imaging Detects Lupus Nephritis With Normal Renal Function. Invest Radiol 54, 89-97, doi:10.1097/RLI.0000000000000511 (2019).
- Reddy, B. S. & Chatterji, B. N. An FFT-based technique for translation, rotation, and scale-invariant image registration. Ieee Transactions on Image Processing 5, 1266-1271, doi:10.1109/83.506761 (1996).
Figures
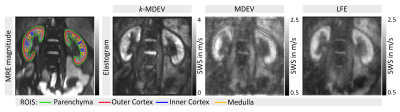
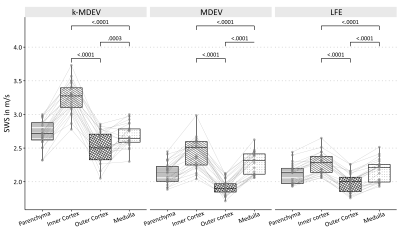
Group statistical plots of regional SWS (shear-wave speed) values of the kidney (averaged over the right and left kidney in each volunteer) as obtained by three different processing pipelines implemented at https://bioqic-apps.charite.de. LFE: local frequency estimation, MDEV: multifrequency dual elasto-visco inversion, k-MDEV: wavenumber-based MDEV.