2236
Free-Breathing MR Elastography of the Kidneys Using TURBINE-MRE1Department of Radiology, Mayo Clinic, Rochester, MN, United States
Synopsis
A free-breathing hybrid radial-cartesian 3D MR elastography technique (TURBINE-MRE) was extended for kidneys with a dual driver system. This technique provides wave images and stiffness maps in a single 3.5-minute free-breathing acquisition. The feasibility of the new technique was demonstrated in a healthy volunteer.
Introduction:
We recently have developed a 3D, hybrid radial-Cartesian, EPI acquisition for MRE (TURBINE-MRE) and demonstrated its application in brain MRE [1, 2] and free-breathing (FB) liver MRE [3]. In this study, we further adapted TURBINE-MRE for kidney MRE on a 3T scanner and demonstrated the feasibility of the technique in a volunteer study.Methods:
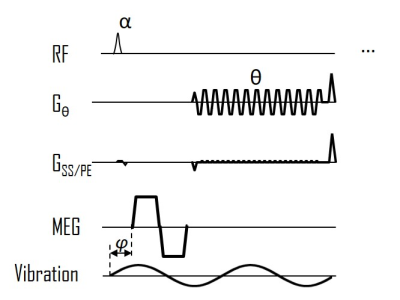
The free-breathing TURBINE-MRE sequence is shown in Figure 1. The EPI blade is rotating continuously with a golden angle of 111.248°. To reduce the echo time and mitigate the strong susceptibility effects at 3T, a bipolar motion-encoding gradient was used with fractional encoding. Two sets of drivers were used in order to deliver sufficient waves in both kidneys.With IRB approval and informed consent, 1 healthy volunteer was scanned on a GE 3T scanner (Signa Premier, GE Healthcare, Waukesha, WI) using a 30-channel flexible anterior array coil and 60-channel embedded posterior array coil (GE Healthcare, Waukesha, WI). The imaging parameters for TURBINE-MRE were TR/TE = 44.4/19.8 ms, FA=16 deg, isotropic resolution = 3.3 mm, FOV = 42x42x21 cm3, 204 EPI blades with a 128 x 64 acquisition matrix, 2x phase-encoding acceleration. The harmonic motions (90-Hz vibration) were encoded by 8.3-ms, 56-mT/m bipolar MEGs (19.5 µm/π-radians encoding efficiency) in 6 motion-encoding directions and 3 time offsets with a total scan time of 3:24 minutes.
As a comparison, standard Cartesian breath-hold (BH) EPI MRE was acquired in the coronal plane with 4.6-mm in-plane resolution, 2.5-mm slice thickness, 28 slices, 6 motion-encoding directions, 4 time offsets and 8 breath-holds of 17 seconds each.
Image Reconstruction: After EPI phase correction, the EPI blades of each MRE repetition were sorted into 6 respiratory states according to the recorded respiratory bellows signal. Then, an XD-GRASP-type reconstruction was performed as described in [3, 4]. The reconstruction was done in Matlab with the adapted open-source code from [5]. MRE stiffness maps were generated by adaptively taking the curl of the 3D displacement fields and performing a 3D direct inversion (DI) algorithm to invert the wavefield as described in the previous study [6, 7].
Results:
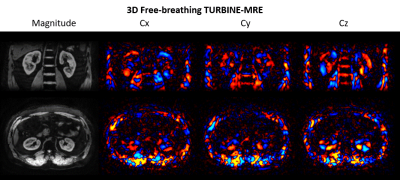
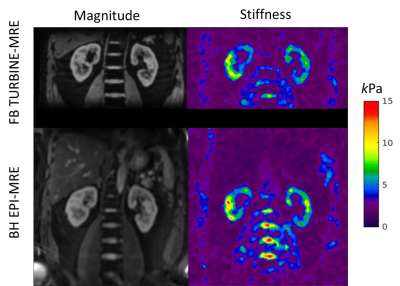
Figure 2 shows animations of the 3D TURBINE-MRE results from the healthy volunteer at end-expiration. MRE magnitude and curl images are presented, reformatted into both coronal and axial views.Figure 3 compares FB TURBINE-MRE and standard BH EPI-MRE magnitude images and stiffness maps. The TURBINE-MRE demonstrates higher in-plane resolution and comparable stiffness maps.
Discussion and Conclusion:
In this study, we have demonstrated the feasibility of the free-breathing TURBINE-MRE sequence for kidney applications at 3T in a healthy volunteer. TURBINE-MRE has been successfully applied for body imaging at 1.5T. However, due to the gradient-echo nature of the sequence, the TURBINE-MRE sequence is prone to susceptibility artifacts at high fields, such as at 3T. In this study, we used a bipolar MEG instead of a 1-2-1 shaped first-moment-nulled MEG. This was helpful to mitigate the susceptibility artifact by reducing the MEG length and TE time while still having sufficient motion sensitivity. Although the bipolar MEG may introduce more phase errors between EPI blades than the first-moment-nulled MEG, these initial results suggest that image quality was reasonable at end-expiration without interblade phase correction. Nevertheless, further investigation will be conducted and a phase correction strategy will be implemented if needed.Acknowledgements
This work was supported by the Center for Individualized Medicine of Mayo Clinic at Rochester, Minnesota.References
1. Sui, Y., et al., TURBINE-MRE: A 3D hybrid radial-Cartesian EPI acquisition for MR elastography. Magn Reson Med, 2021. 85(2): p. 945-952.
2. Arani, A., et al. Sparse Reconstruction Strategy for Highly-Accelerated 3D TURBINE Magnetic Resonance Elastography. in ISMRM Workshop on Data Sampling & Image Reconstruction. 2020. Sedona, AZ.
3. Sui, Y., et al. Respiratory Motion-Resolved Free-Breathing MR Elastography Of Liver. in ISMRM. 2021.
4. Feng, L., et al., XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med, 2016. 75(2): p. 775-88.
5. XD-GRASP Matlab Code. Available from: https://cai2r.net/resources/software/xd-grasp-matlab-code.
6. Manduca, A., et al., Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Medical Image Analysis, 2001. 5(4): p. 237-254.
7. Murphy, M.C., et al., Measuring the characteristic topography of brain stiffness with magnetic resonance elastography. PLoS One, 2013. 8(12): p. e81668.
Figures