2230
Three-dimensional reconstruction and characterization of bladder deformations1Aix Marseille Univ, Universite de Toulon, CNRS, LIS, Marseille, France, 2Aix Marseille Univ, CNRS, CRMBM, Marseille, France
Synopsis
Pelvic floor disorders are prevalent diseases and only 2D dynamic observations of straining exercises at excretion are available in clinics. The understanding of three-dimensional pelvic organs mechanical defects is not yet achievable. We proposed a complete methodology for the 3D representation of the bladder combined with 3D representation of the location of the highest strain areas on the organ surface. We assessed the potential of our method on eight control subjects for the reconstruction of bladder during intense straining from forced breathing exercises. The proposed methodology provided, for the first time, a proper 3D+t spatial tracking of bladder non-reversible deformations.
Introduction
Pelvic floor disorders affect one fourth of adult women and the prevalence increases with age, up to 50% for women older than 80 years1,2. These pathologies are mainly characterized by a weakening of the pelvic floor muscles leading to mechanical dysfunction of the pelvic organs support structures3. Magnetic resonance imaging (MRI) has emerged as one of the most attractive non-invasive methods for pelvic diagnosis4 and guidelines emphasize the importance of dynamic MRI examinations to identify pathological organs deformations5. Current clinical practice involves 2D dynamic MRI acquiring a single sagittal plane during straining exercises involving excretion. The visualization and characterization of pelvic dynamics by MRI has so far only been studied in the midsagittal plane. An understanding of three-dimensional deformities could provide full assessment of the pelvic organ mechanical defects. Due to speed limitation in MRI acquisition, 3D imaging have been exclusive to static pelvic MRI so far, to observe anatomy at rest during instructed apnea (Fig. 1). 3D dynamic MRI techniques are commonly associated with cardiovascular or abdominal imaging where k-space can be segmented over multiple cycles of repeated deformations6. Such approaches are not applicable for pelvic imaging during organ loading exercises because there is no repetition. The extreme nature of the pelvic organ movements induced during these exercises requires an order of magnitude faster imaging (< 1 s) and prevents their reproducibility.Methods
We combined up-to-date multi-planar 2D dynamic MRI techniques with image processing approaches to reconstruct 3D dynamic volumes of pelvic organs under loading exercises. This study is a complete extension of a previous seminal work7. First, we have extended the acquisition process by introducing three geometrical configurations of dynamic multi-slices acquisition immediately transferable to the clinics (Fig. 2), preceded by a 3D static acquisition used to generate a subject-specific model. Second, we leveraged a semi-automated segmentation method, based on a fusion of image registration approaches, for the tracking of organs in the dynamic planes, regardless of their spatial orientation. Third, linear interpolations on the geodesic path of each multi-planar segmentation have been proposed to refine the time scale inherited from the acquisition methods (Fig. 3). Finally, we built dynamic 3D representations of organs through non-linear geometric registrations between each dynamic partial volumes and the complete static volume of the high-resolution static acquisition (Fig. 4). Furthermore, the organ reconstruction process allowed a direct characterization of the 3D deformations undergone by the pelvic organs during loading exercises. Analysis of the deformation fields allowing the reconstruction of organs, and more particularly the study of the resulting Jacobians, revealed local volume changes over time and provided a high-level 3D representation of the location of the highest strain areas on the organs surface.Results
Our method was evaluated on 8 healthy individuals. Since no extrageneous liquid was injected into pelvic cavities in this study, only the segmentation of the bladder was possible, and the analysis focused exclusively on this organ. During three 1:20 minute forced breathing exercises, subjects alternately inhaled and exhaled at maximum capacity to increase intra-abdominal pressure, causing pelvic organ deformities. For each subject, depending on the configurations, 400 to 680 volumes were reconstructed at a rate of 5 to 9 volumes per second. Validation of the 3D organ reconstructions was a difficult process due to the lack of ground truth as a point of comparison. The 3D reconstructions were therefore validated via the coherence of the reconstructed volumes with respect to the accuracy of the registration (average and Hausdorff distance between the dynamic skeletons and the reconstructed dynamic volumes of 0.4 mm and 2.2, respectively) and the conservation of the volume of the incompressible bladder (average value of deviation relative to the static volume of approximately 2.5%).Discussion
Little work has been done on the analysis of the pelvic organ dynamics. Recently, Courtecuisse et al. has proposed a registration approach to deform pelvic organs acquired in 3D static acquisition using single plane 2D dynamic acquisition8. However, biomechanical knowledge had to be injected into the registration model as the information from a single 2D slice is not enough to accurately guide organs that deform nonlinearly in 3D. In our study, we provided 3D spatial coverage of the organs through the acquisition of several dynamic slices and dynamic volumes were reconstructed independently of any mechanical model. Furthermore, our processing pipeline proved to be compatible with all three configurations in terms of bladder reconstruction and deformation characterization, with equivalent end results (Fig. 5).Conclusion
To our knowledge, this study is the first to propose both dynamic 3D visualization of the pelvic region during exercise and a dynamic 3D tracking of the organs. Our method is directly applicable in the clinic as associated to a rapid MRI protocol (≈ 2 min). In future clinical studies, the choice of the multiplanar configuration could be adapted to the patient and to the pelvic static disorder studied. This ongoing work could allow a systemic characterization of pelvic organ deformity9 and better clinical management of pelvic floor disorders. Future studies will be conducted on patients with ethical permission to inject extragenic fluid into the rectal and vaginal cavities so that all pelvic organs can be segmented.Acknowledgements
No acknowledgement found.References
1. Nygaard, M. D. Barber, K. L. Burgio, K. Kenton, S. Meikle,J. Schaffer, C. Spino, W. E. Whitehead, J. Wu, D. J. Brody,et al.,“Prevalence of symptomatic pelvic floor disorders in US women,” Jama,vol. 300, no. 11, pp. 1311–1316, 2008.
2. J. M. Wu, C. P. Vaughan, P. S. Goode, D. T. Redden, K. L. Burgio, H. E.Richter, and A. D. Markland, “Prevalence and trends of symptomatic pelvic floor disorders in us women,” Obstetrics and gynecology, vol. 123,no. 1, p. 141, 2014.
3. J. E. Jelovsek, C. Maher, and M. D. Barber, “Pelvic organ prolapse,” The Lancet, vol. 369, no. 9566, pp. 1027–1038, 2007.
4. C. A. Woodfield, S. Krishnamoorthy, B. S. Hampton, and J. M. Brody,“ Imaging pelvic floor disorders: trend toward comprehensive mri,” American Journal of Roentgenology, vol. 194, no. 6, pp. 1640–1649, 2010.
5. R. F. El Sayed, C. D. Alt, F. Maccioni, M. Meissnitzer, G. Masselli, L. Manganaro, V. Vinci, D. Weishaupt, ESUR, E. P. F. W. Group, et al., “Magnetic resonance imaging of pelvic floor dysfunction-joint recommendations of the esur and esgar pelvic floor working group,” European radiology, vol. 27, no. 5, pp. 2067–2085, 2017.
6. L. Feng, L. Axel, H. Chandarana, K. T. Block, D. K. Sodickson, and R. Otazo, “Xd-grasp: golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing,” Magnetic resonance in medicine, vol. 75, no. 2, pp. 775–788, 2016.
7. A. C. Ogier, S. Rapacchi, A. Le Troter, and M.-E. Bellemare, “3D dynamic MRI for pelvis observation - a first step,” in 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), pp. 1801–1804, IEEE, 2019.
8. H. Courtecuisse, Z. Jiang, O. Mayeur, J. Witz, P. Lecomte-Grosbras, M. Cosson, M. Brieu, and S. Cotin, “Three-dimensional physics-based registration of pelvic system using 2D dynamic magnetic resonance imaging slices,”Strain, p. e12339, 2020.
9. K. Makki, A. Bohi, A. C. Ogier, and M.-E. Bellemare, “A new geodesic-based feature for characterization of 3D shapes: application to soft tissue organ temporal deformations,” in 2021 25th International Conference on Pattern Recognition, IEEE, 2021
Figures
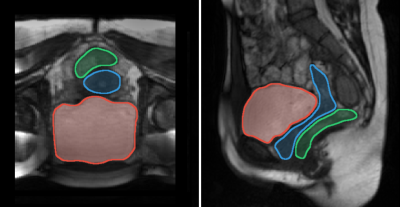
Fig. 1: Axial (left) and sagittal (right) views of pelvic floor acquired during a maximum expiration apnea with delineations of the major pelvic organs: bladder (red), uterus/vagina (blue), and rectum (green).
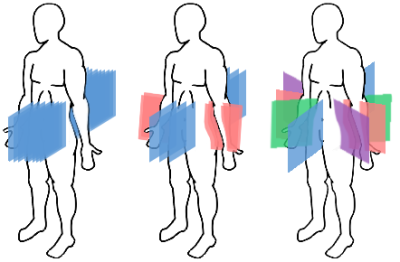
Fig. 2: Geometrical configuration of the planes for the three dynamic multi-planar acquisitions sequences.
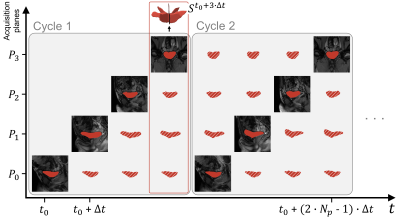
Fig. 3: Spatio-temporal configuration of the acquisition planes and temporal reconstruction scheme, illustrated for Cstar. Filled segmentations refer to instants for which an image was acquired. Hatched segmentations correspond to the temporally reconstructed ones. All segmentations of all acquisition planes of a given instant t are combined to provide the skeleton St of the organ volume at that time.
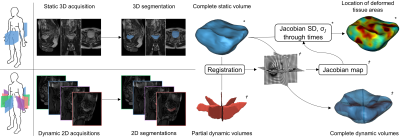
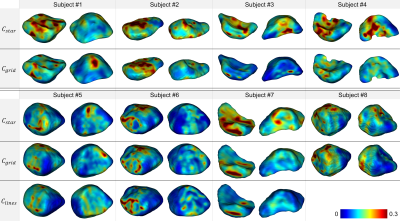
Fig. 5: Anterior (left) and posterior (right) views for each subject and geometrical configuration of the σJ map projected on the corresponding reference static volumes. Reddest areas indicate the location of the highest strain areas on the organ surfaces during dynamic acquisition sequences. Frequency of pushing phases and magnitude of the load applied to the organs were highly variable between each configuration but, for all subjects, the spatial distribution of deformed tissue regions was similar between configurations.