2222
Use of 7 Tesla MRI for frameless pre-surgical planning with skin-adhesive fiducials1Radiology, Mayo Clinic, Rochester, MN, United States
Synopsis
This study aimed to investigate the use of 7 Tesla MRI for frameless pre-surgical planning for tumor resection applications. Image geometric distortions at the locations of 10 skin-adhered fiducials were minimized in 7 Tesla images, and compared to in vivo results from a current 3 Tesla frameless imaging protocol. Geometric accuracy of < 1 mm was obtained in 7 Tesla images at the surface of a head-shaped phantom.
Introduction
7T MRI offers significant benefits in terms of spatial and contrast resolution compared to imaging at lower field strengths1,2. The superior image quality can better delineate the targets of many interventional neurosurgical procedures such as the basal ganglia regions which are targeted for deep brain stimulation surgery3, and tumors for surgical resection or radiation therapy4. However, the potential for increased image geometric distortions has impaired the use of 7T MRI for these applications, casting doubt on the fidelity of the planning data5. Such distortions can arise from system-level sources such as nonlinearity of the magnetic field gradients and inherent B0 inhomogeneity6, which occur at all field strengths. However, patient-specific sources of distortion such as increased magnetic susceptibility-induced B0 inhomogeneities and chemical shift artifacts are worse at higher fields, and the initial worry was that they would prevent the use of 7T MRI for planning purposes where high geometric accuracy is of paramount importance7.To overcome this limitation, CT images are acquired and registered to the MR images, thereby ensuring geometrical accuracy. This exposes the patient to an ionizing radiation dose and further requires a second imaging examination for the patient, with associated additional expense. Furthermore, in applications where a stereotactic frame is not used, e.g. in pre-surgical planning for tumor resection, the registration from MRI to operating room space is performed using 10 skin-mounted fiducials placed around the patients head. The location of such fiducials close to areas where susceptibility and chemical shift effects are greatest is a further cause for concern at 7T.
The aim of this study was to investigate the use of 7T MRI for pre-surgical planning for frameless applications. Image geometric distortions at the locations of 10 skin-mounted fiducials were minimized in 7T images via sequence optimization, and compared to results from a current 3T frameless imaging protocol.
Methods
An oil-filled 3D grid phantom (150mm diameter, 140mm high; Figure 1) was developed to perform a fine calibration of the 7T system’s gradients. The phantom’s ground truth dimensions were determined via high-resolution CT imaging (Somatom Force, Siemens). Baseline MR images of the phantom were acquired using a high receiver-bandwidth sequence, repeated with different frequency-encoding directions; the average distance measurements between these acquisitions were used. An oil-filled head-shaped phantom with an added surface fat layer was also imaged (CT and MR) with the fiducials in situ.Three volunteers were imaged (with informed consent) consecutively at 3T (Prisma, Siemens) and 7T (Terra, Siemens). 10 skin-adhesive fiducials (IZI Medical Products, USA) were placed on the subject’s exposed skin surface at standard placement locations for frameless procedures (specifically: nasion, superior mid frontal, and bilateral mastoid, zygoma, and frontal areas). Care was taken to avoid touching the fiducials when positioning the subjects in the head coils, per standard protocol. The imaging protocol was comprised MPRAGE, T2 SPACE and T2 SPACE FLAIR acquisitions; sequence details are presented in Figure 2. The vendor’s 3D distortion correction algorithm was used in each case. An additional GRE fieldmap was acquired on both scanners using a multi-echo GRE sequence with TE1/2 = 4.76/9.52 ms at 3T, and TE1/2 = 1.14/3.14 ms at 7T. Custom code in Matlab was used to perform distortion correction of the images using the fieldmap data. Fiducial localization was performed using 3D Slicer (www.slicer.org), with absolute fiducial positioning errors determined in phantom experiments following registration to the CT images. For human experiments, 3T and 7T images were registered and relative differences in fiducial locations were determined. A two-tailed paired t-test was used to detect statistically significant differences in each case.
Results
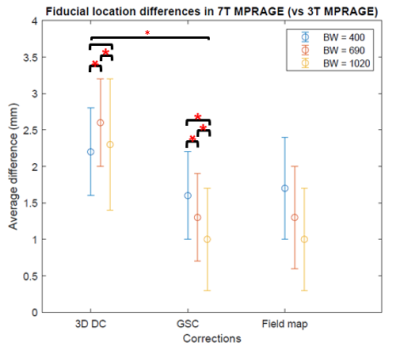
Phantom measurements at 7T revealed distance scaling errors of 1.1%, 2.2% and 1.0% along the x-, y- and z-axes respectively. These system mis-calibrations were traced back to deviations from sphericity of the scanner’s vendor calibration phantom. A global gradient scaling correction (GSC) factor was applied to correct for this; the effects of this correction factor for one subject’s images are shown in Figure 3. Geometric distortion (MR compared to ground truth CT data) was <1mm for the 7T images acquired with 1020Hz/pixel BW.Data for the relative fiducial location measurement differences between 3T and 7T subjects are presented in Figure 4. The GSC factor and higher receiver bandwidth had the most impact on the fiducial location accuracy, while the fieldmap correction effected no change. Figure 5 shows corresponding the SPACE and FLAIR data.
Discussion & Conclusions
Highly accurate 7T images with <1mm geometric distortion compared to CT images, were obtained with an optimized imaging sequence. Fieldmap corrections were found to be unnecessary, once the GSC was applied and high receiver bandwidth images used. Judicious placement of fiducials is still warranted to avoid potential for skin movement, or dislodgement due to the close-fitting 7T head coil. Furthermore, locations low in the head should be avoided due to the signal drop-off at 7T (due to B0 and B1 issues, and head coil limitations).In conclusion, 7T MRI can be used to perform frameless pre-surgical planning with skin-adhesive fiducials with slight modifications to standard imaging sequences (using high receiver bandwidth) and provided the system gradient calibrations are checked. Fiducial location is also an important consideration.
Acknowledgements
No acknowledgement found.References
1. Fagan AJ, Welker KM, Amrami KK, et al. Image Artifact Management for Clinical Magnetic Resonance Imaging on a 7 T Scanner Using Single-Channel Radiofrequency Transmit Mode. Invest Radiol 2019;54:781-91.
2. Ladd ME, Bachert P, Meyerspeer M, et al. Pros and cons of ultra-high-field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc 2018;109:1-50.
3. Rusheen A, Goyal A, Owen R, et al. The development of ultra-high field MRI guidance technology for neuronavigation. Journal of Neurosurgery 2021;accepted.
4. Peerlings J, Compter I, Janssen F, et al. Characterizing geometrical accuracy in clinically optimised 7T and 3T magnetic resonance images for high-precision radiation treatment of brain tumours. Phys Imaging Radiat Oncol 2019;9:35-42.
5. Voormolen EH, Diederen SJH, Woerdeman P, et al. Implications of Extracranial Distortion in Ultra-High-Field Magnetic Resonance Imaging for Image-Guided Cranial Neurosurgery. World Neurosurg 2019;126:e250-e8.
6. Lau JC, Khan AR, Zeng TY, MacDougall KW, Parrent AG, Peters TM. Quantification of local geometric distortion in structural magnetic resonance images: Application to ultra-high fields. Neuroimage 2018;168:141-51.
7. Duchin Y, Abosch A, Yacoub E, Sapiro G, Harel N. Feasibility of using ultra-high field (7 T) MRI for clinical surgical targeting. PLoS One 2012;7:e37328.
Figures