2219
Multi-modal assessment of dose-related changes in regional lung function in non-small cell lung cancer patients receiving radiotherapy1Department of Oncology and Metabolism, The University of Sheffield, Sheffield, United Kingdom, 2POLARIS, Department of Infection, Immunity and Cardiovascular Disease, The University of Sheffield, Sheffield, United Kingdom, 3Insigneo Institute for in silico medicine, The University of Sheffield, Sheffield, United Kingdom, 4Clinical Oncology, Weston Park Cancer Centre, Sheffield, United Kingdom, 5Radiotherapy Physics, Weston Park Cancer Centre, Sheffield, United Kingdom
Synopsis
Radiotherapy plays a central role in the management of lung cancer. However, despite advances, survival of lung cancer patients undergoing radiotherapy remains poor, partly attributable to the incidence of radiation-induced lung injury, which is exacerbated in patients with poor pulmonary function. Both ventilation and perfusion information are vital to providing a complete picture of pulmonary function, ideally in a regional fashion. Here, we develop and apply an image acquisition and analysis pipeline to assess dose-related changes in regional lung function as derived from hyperpolarized gas ventilation and dynamic contrast-enhanced perfusion MRI in lung cancer patients receiving a course of radiotherapy.
Introduction
Radiotherapy (RT) plays a central role in the management of non-small cell lung cancer (NSCLC). Despite advances in RT, including the development of stereotactic ablative RT (SABR), survival outcomes remain disappointing.One of the factors underlying poor survival is radiation-induced lung injury, which is exacerbated by poorer functional pulmonary reserve pre-RT1. The pre-RT lung function of NSCLC patients is currently assessed by conventional pulmonary function tests (PFTs). However, PFTs are relatively crude as they provide global, whole-lung measures and have limited sensitivity to early changes2.
As the primary function of the lungs is gas exchange, both ventilation (V) and perfusion (Q) information are vital to providing a complete picture of lung function, preferably in a regional fashion. Despite poor spatial and temporal resolution, regional lung function has traditionally been assessed by radionuclide scintigraphy or single-photon emission computed tomography3. Alternatively, MRI can provide high-resolution images of regional lung function as assessed by dynamic contrast-enhanced (DCE) perfusion proton (1H) MRI and hyperpolarized gas ventilation MRI4.
Here, we develop and apply an image acquisition and analysis pipeline to assess dose-related changes in regional lung function as derived from MRI V/Q in NSCLC patients receiving a course of RT.
Methods
Patient and imaging data12 NSCLC patients receiving RT underwent pre-RT free-breathing CT as standard-of-care and inspiratory breath-hold CT at functional residual capacity (FRC)+1L. Helium-3 (3He) (3D SSFP) and 1H-MRI (3D SPGR) were acquired in the same breath and inflation state as inspiratory CT5 following inhalation of 200ml hyperpolarized 3He and 800ml N2. Imaging was performed at 1.5T (GE HDx) using a 3He transmit-receive vest coil (CMRS). Patients were repositioned in an 8-channel cardiac coil with DCE-MRI acquired using a 3D SPGR sequence and parallel imaging6. All MRI scans were repeated 3-4 months post-RT.
Image registration
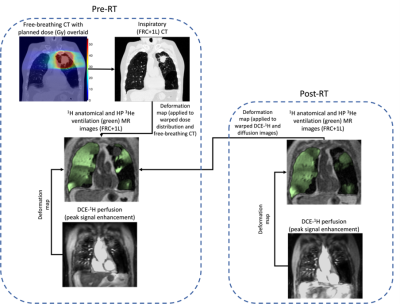
Images were segmented using previously described methods7,8. Free-breathing CT, planned dose distribution and RT contours were deformably registered to inspiratory CT. Pre-RT inspiratory CT, the warped dose distribution and RT contours, post-RT 3He-MRI and pre- and post-RT DCE-MRI were registered to the spatial domain of pre-RT 3He-MRI9. The registration workflow is shown in Figure 1.
Dosimetric and image analysis
A threshold of ≥20Gy was applied to the warped dose distribution minus the gross tumour volume. This threshold was selected as it has been validated as a predictive marker for radiation pneumonitis10. All lung function metrics described below were computed in this dose region.
Ventilation and perfusion defect percentages (VDP, QDP, respectively) were computed from the ratios of the ventilated and perfused lung segmentations over the pre-RT 1H-MRI thoracic cavity volume. The spatial overlap of ventilated and perfused defects was computed via the Dice similarity coefficient (DSC) to assess global V/Q matching (DSC(V/Q)). Fractional ventilation (FV)11, pulmonary blood volume (PBV), pulmonary blood flow (PBF) and mean transit time (MTT)12 were computed as previously described. The V/Q ratio (FV/PBV) was used as a surrogate of gas exchange13.
Statistical analysis
Wilcoxon signed-rank tests were used to assess differences in imaging biomarkers pre- and post-RT.
Results
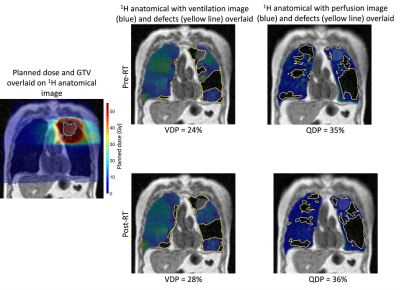
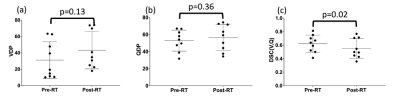
9 of the 12 patients underwent all imaging tests required to be included in this study. Figure 2 shows an example of the workflow shown in Figure 1 applied successfully to a representative patient in the study. Changes in VDP and QDP can be observed, particularly in the vicinity of the tumor, which receives the highest doses of radiation.Figure 3 summarizes the VDP, QDP and DSC(V/Q) results for all patients pre- and post-RT. Statistically significant differences between these metrics before and after RT were only observed for DSC(V/Q).
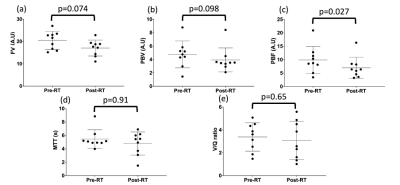
Figure 4 summarizes the results for the V/Q metrics. Although PBF was the only metric to exhibit a statistically significant difference between pre- and post-RT, trends towards significance were observed for FV and PBV.
Discussion
No hitherto published study in humans has investigated dose-related changes in MRI V/Q. Our acquisition and analysis pipeline allowed these investigations to be conducted and showed significant changes in the DSC(V/Q), indicating that combined V/Q may be effective in detecting radiation-induced lung injury.The significant changes in vascular response to radiation as observed by pre- and post-RT PBF measurements are in line with preclinical studies in irradiated rats14-15; radiation induces endothelial injury which exposes basement membrane, resulting in platelet adhesion and vascular obstruction, thus limiting blood flow15. Although no statistically significant differences were observed in other regional lung function biomarkers pre- and post-RT, this is likely attributable to the relatively small sample size and range of pre-existing pulmonary functional reserve of the NSCLC patients in this study. Moreover, the dose threshold of ≥20Gy selected in this study may have masked differences in higher dose regions. Whilst there is a strong evidential basis for this threshold, a dose-binning approach may be more appropriate. Further work will investigate stratifying patients according to pre-RT lung function and determining if regional lung function changes are associated with low, moderate and high doses.
Conclusion
Quantitative analysis of dose-related changes in MRI V/Q-derived biomarkers is feasible using a dedicated acquisition and analysis protocol. Significant dose-related reduction in the overlap of ventilation and perfusion post-RT indicates that MRI V/Q may play a role in detecting radiation-induced lung injury in patients undergoing thoracic RT.Acknowledgements
This work was supported by Yorkshire Cancer Research, Sheffield Hospitals Charity, Weston Park Cancer Charity, National Institute of Health Research and the Medical Research Council.References
1. Robnett TJ, Machtay M, Vines EF, et al. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000;48:89-94.
2. Que C, Cullinan P, Geddes D. Improving rate of decline of FEV1 in young adults with cystic fibrosis. Thorax. 2006;61:155-7.
3. Marks LB, Spencer DP, Bentel GC, et al. The utility of SPECT lung perfusion scans in minimizing and assessing the physiologic consequences of thoracic irradiation. Int J Radiat Oncol Biol Phys. 1993;26(4):659-68.
4. Ireland RH, Din OS, Swinscoe JA, et al. Detection of radiation-induced lung injury in non-small cell lung cancer patients using hyperpolarized helium-3 magnetic resonance imaging. Radiother Oncol. 2010;97(2):244-8.
5. Tahir BA, Hughes PJC, Robinson SD, et al. Spatial comparison of CT-based surrogates of lung ventilation with hyperpolarized helium-3 and xenon-129 gas MRI in patients undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 2018;102(4):1276-86.
6. Johns CS, Swift AJ, Rajaram S, et al. Lung perfusion: MRI vs. SPECT for screening in suspected chronic thromboembolic pulmonary hypertension. 2017;46(6):1693-7.
7. Hughes PJC, Horn FC, Collier GJ, et al. Spatial fuzzy c-means thresholding for semiautomated calculation of percentage lung ventilated volume from hyperpolarized gas and 1H MRI. J Magn Reson Imaging. 2018;47(3):640-6.
8. Schiwek M, Triphan SMF, Biederer J, et al. Quantification of pulmonary perfusion abnormalities using DCE-MRI in COPD: comparison with quantitative CT and pulmonary function. Eur Radiol. 2021. DOI: 10.1007/s00330-021-08229-6
9. Tahir BA, Swift AJ, Marshall H, et al. A method for quantitative analysis of regional lung ventilation using deformable image registration of CT and hybrid hyperpolarized gas/1H MRI. Phys Med Biol. 2014;59(23):7267-77.
10. Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85(2):444-50.
11. Tzeng YS, Lutchen K and Albert M. The difference in ventilation heterogeneity between asthmatic and healthy subjects quantified using hyperpolarized 3He MRI. J Appl Physiol. 2009;106(3):813-22.
12. Sourbron SP and Buckley DL. Classic models for dynamic contrast-enhanced MRI. NMR Biomed. 2013:26(8):1004-27.
13. Hughes PJC, Tahir BA, Horn FC, et al. Quantitative ventilation-perfusion imaging using co-registered hyperpolarized gas and contrast enhanced 1H perfusion MRI. ISMRM 2017.
14. Peterson LM, Evans ML, Graham MM, et al. Vascular response to radiation injury in the rat lung. Radiation Research. 1992:129(2):139-48.
15. Peterson LM, Evans ML, Thomas KL, et al. Vascular response to fractionated irradiation in the rat lung. Radiation Research. 1992:132(2):224-6.
Figures