2217
On-board pseudo-MRI guidance for prostate radiotherapy on a conventional X-ray guided LINAC1Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
Current target localization for prostate radiotherapy on a conventional LINAC consists of acquiring a cone-bean CT (CBCT) image and matching it to implanted fiducial markers inside the prostate due to poor soft-tissue contrast of CBCT. MRI provides better soft tissue contrast and no ionization radiation, but there are very few MR-LINACs, and conventional LINACs with x-ray imaging capabilities are much more ubiquitous. This study proposes to use prior MRI acquisitions, PCA motion modeling, and on-board CBCT from a conventional LINAC to obtain on-board pseudo-MRI for target localization in prostate radiotherapy.
Background
The gold standard for target localization in prostate radiotherapy is on-board cone-beam CT (CBCT), but soft tissue contrast in CBCT is poor, so often times gold fiducial markers are implanted into the prostate to be utilized for on-board imaging and target localization [1]. MRI offers superior soft tissue contrast in the prostate region and no ionizing radiation compared to CT and CBCT. The combined MRI-LINAC system offers capabilities for MRI-guided radiotherapy for prostate cancer, but few clinics have an MRI-LINAC due to the high cost. Conventional LINACs with x-ray capabilities are much more common, and the ability to generate on-board pseudo-MRI images using a conventional LINAC would substantially improve target localization accuracy for prostate radiotherapy and potentially eliminate the need for invasive fiducial implantation. A previous study used prior 4D MRI, motion modeling, and on-board kV projections from a conventional LINAC to generate on-board pseudo-4D-MRI to improve target localization in liver radiotherapy [2]. Other studies have modeled prostate motion on the basis on Principal Component Analysis (PCA) using multiple CTs acquired at various time points to analyze the intrafractional motion and deformation of the prostate, rectum, and bladder [3, 4]. The purpose of this study is to use prior MRI images, PCA-motion modeling, and on-board CBCT to estimate on-board pseudo-MRI for prostate radiotherapy target localization.Methods
The overall workflow is shown in Figure 1.Prior MRI data: Multiple 3D MRI acquisitions were performed during the simulation process (MRI in the treatment position) to represent the patient anatomy and motion (bladder filling and prostate position) at various timepoints throughout a 30-minute period. One MRI was selected as the reference MRI (MRIref) and a synthetic CT (sCTref) was generated [5] from the MRIref.
Motion modeling: Deformable image registration was performed from the MRIref to all other MRIs. PCA was applied to the DFMs and the DFM was considered a weighted linear combination of the principal motion modes.
Pseudo-MRI estimation using the on-board CBCT: The weighting coefficients from PCA motion modeling were optimized based on minimizing the difference between the deformed and the on-board CBCT. The estimated on-board pseudo-MRI is considered a deformation of the MRIref and the goal was to solve for the deformation field map (DFM) that best estimates the on-board pseudo-MRI based on the deformed sCTref and the on-board CBCT acquired during patient set-up.
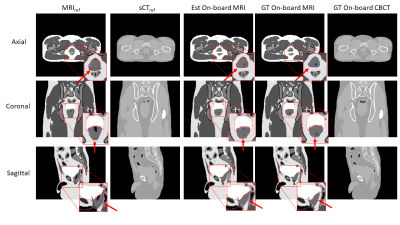
Numerical phantom and prostate patient studies: The method was evaluated using an anthropomorphic XCAT phantom simulating various bladder volume and prostate motion based on literature [6] and a prostate patient data. Table 1 shows the patient imaging properties and Table 2 shows the XCAT simulation properties and two on-board scenarios for the study: one with 6mm motion magnitude and 20% bladder volume increase and a second one with 10mm motion magnitude and 33% bladder volume increase. For the patient data, all images were post processed to 1.03mm x 1.03mm x 2mm resolution and image size 256 x 256 x 86. For the XCAT data, the resolution was 1.88mm x 1.88mm x 3mm and image size was 256 x 256 x 102. For the XCAT data, the bladder and prostate structures were contoured in the estimated and ground-truth on-board MRIs for quantitative analysis. Volume difference was calculated for the bladder structure and center of mass shift (COMS) was calculated for the prostate structures. The workflow was tested for the patient data, and the COMS of the fiducial markers between the estimated on-board pseudo-MRI and the on-board CBCT was calculated.
Results
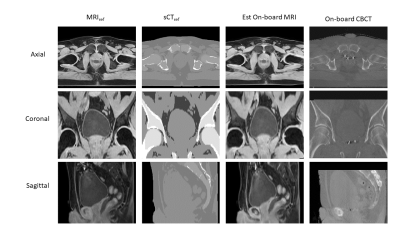
For the XCAT data, the volume difference between ground truth and estimated bladder contours was 1.28% and 7.85% for the two XCAT scenarios, respectively. The COMS between ground truth and estimated prostate contours was 1.37 and 1.31mm for the two XCAT scenarios, respectively. Figure 2 shows the MRIref, sCTref, estimated on-board MRI, ground-truth on-board MRI and ground-truth on-board CBCT for XCAT scenario 2, which corresponds to 33% increase in bladder volume and prostate position shift of 8mm and 6mm in the anterior-posterior (AP) and superior-inferior (SI) directions, respectively, from simulation to treatment. The estimated and ground-truth bladder and prostate contours are shown in Figure 2, as well. For the patient data, the COMS between the estimated on-board MRI and the acquired on-board CBCT were 2.30mm, 1.45mm, and 2.30mm for the 3 fiducials separately. Figure 3 shows MRIref, sCTref, estimated on-board MRI, and ground-truth on-board CBCT for the prostate patient.Conclusions
Preliminary results suggest that prior MRI from simulation, motion modeling, and on-board CBCT acquired for patient set-up can be used to estimate a pseudo-MRI for on-board target localization in prostate radiotherapy using a conventional LINAC with only x-ray imaging capabilities. This method can be expanded to deform other MRI-contrast images acquired during simulation to achieve multiple estimated on-board pseudo-MRIs with varying contrast. The proposed method may result in eliminating the need to invasively implant fiducial markers for prostate localization, since the prostate and other soft tissue contrast is substantially improved in MRI compared to CBCT.Acknowledgements
No acknowledgement found.References
[1] O’Neill, A., Jain, S., Hounsell, A., O’Sullivan, J., “Fiducial marker guided prostate radiotherapy: a review,” Br. J. Radiol. 2016 Dec;89(1068):20160296
[2] Harris, W., Wang, C., Yin, FF., Cai, J., Ren, L., “A Novel method to generate on-board 4D MRI using prior 4D MRI and on-board kV projections from a conventional LINAC for target localization in liver SBRT” Med. Phys. 2018 Jul; 45(7):3238-3245
[3] Sohn, M., Birkner, M., Yan, D., Alber, M., “Modelling individual geometric variation based on dominant eigenmodes of organ deformation: implementation and evaluation” Phys. Med. Biol. 2005 Dec 21;50(24):5893-908
[4] Sohn, M., Sobotta, B., Alber, M., “Dosimetric treatment course simulation based on a statistical model of deformable organ motion,” Phys. Med. Biol. 2012 Jun 21;(57)12:3693-709
[5] Tyagi, N., Fontenla, S., Zelefsky, M., Chong-Ton, M., Ostergren, K., Shah, N., Warner, L., Kadbi, M., Mechalakos, J., Hunt, M., “Clinical workflow for MR-only simulation and planning in prostate” Radiat. Oncol. 2017 Jul 17;12(1):119
[6] Pommer, T., Hun Oh, J., Rosenshold, M., Deasy, J., “Simulating intrafraction prostate motion with a random walk model” Adv. Radiat. Oncol. 2017 Jul-Sep;2(3):429-436
Figures