2214
Data-driven optimization of intravoxel incoherent motion imaging for clinical endpoints in radiotherapy on a 1.5 T MR-Linac1Joint Department of Physics, The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, London, United Kingdom, 2Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 3Department of Radiation Oncology, The Netherlands Cancer Institute, Amsterdam, Netherlands, 4Physical Sciences Platform, Sunnybrook Research Institute, Toronto, ON, Canada, 5Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 6Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 7Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
Measuring the detectable effect size is crucial for setting up clinical trials involving quantitative MRI for treatment adaptation, response assessment and outcome prediction in MR-guided radiotherapy. For four different tumor sites (brain, head and neck, prostate and rectum) diffusion-weighted MRI protocols were optimized for intravoxel incoherent motion imaging based on minimizing the mean relative error of the IVIM parameters. For full IVIM model fits, 4-5 b-values were found optimal, while a fit with fixed D* was best performed with 3 b-values. MR-Linac systems currently have limitations regarding gradient performance and number of coil channels and qMRI techniques require careful optimization.
Purpose
Measuring the detectable effect size is crucial for setting up clinical trials involving quantitative MRI (qMRI) biomarkers and for adequate interpretation of daily qMRI measurements in the context of treatment adaptation, response assessment and outcome prediction. The aim of this study is to suggest a data-driven approach to characterize and optimize diffusion-weighted (DW) magnetic resonance imaging (MRI) for intravoxel incoherent motion (IVIM) analysis on hybrid MR-Linacs.Introduction
The IVIM model1 introduces a perfusion compartment, which contributes the perfusion fraction f to the unweighted signal. In addition to the tissue diffusion coefficient D, a pseudo-diffusion coefficient D* is used to describe perfusion-related signal attenuation. All IVIM parameters have shown promise as biomarkers for assessing tumor cellularity and early response to radiation therapy2,3,4,5, but variability in estimates of quantitative imaging biomarkers (QIBs) in longitudinal studies can lead to uncertainty in detecting post-treatment changes. In particular for multi-center trials, repeatability tests are essential to identify sources of errors across clinical sites. Substantial differences in the design of an MR-Linac6 compared to conventional MRI scanners make it challenging to optimize DW MRI protocols5,7. Here we propose a data-driven strategy based on a set of calibration scans to optimize the acquisition, such that uncertainty in the IVIM parameter estimates is minimized and quantified to facilitate robust interpretation regarding clinical end-points.Methods
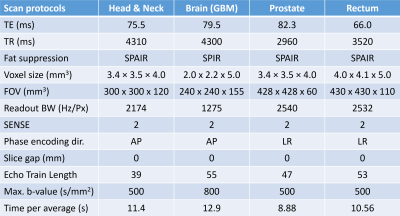
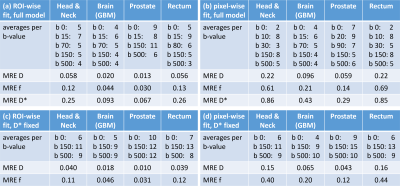
DW data were acquired using single-shot EPI in 6 patients (1 head & neck cancer, 3 glioblastoma multiforme (GBM), 1 prostate carcinoma and 1 rectal carcinoma) across different 1.5T MR-Linac (Elekta AB, Stockholm) sites. Patients consented to participate in clinical studies approved by local IRBs. Calibration scan parameters (Table 1) followed consensus guidelines for DWI on the Unity system7 and consisted of single-average trace-weighted DW images for a wide range of b-values and were repeated five times within one session.Monte-Carlo simulations were performed using the average per-pixel standard deviation of the signal intensity within the delineated ROI. To limit search space, allowed b-values were restricted to 0, 2, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 60, 70, 80, 90, 100, 125, 150, 175, 200, 250, 300, 350, 400, 450 and 500 s/mm2. Starting from 25 averages for each of the 27 allowed b-values, in each step one of the following two actions was performed: Either one average was removed from one b-value or one b-value was removed completely and its averages minus one were distributed randomly among other b-values. For each of these possibilities 2500 IVIM fits were performed on data simulated according to $$$S(b)=S_0[(1-f)e^{-bD}+fe^{-bD^*}]$$$ by drawing ground truth signal intensity S0, D, f and D* randomly from $$$S_0\in[0.4,5]$$$, $$$f\in[0.01, 0.4]$$$, $$$D\in[0.8, 3.0]\times10^{-3}mm^2/s$$$ and $$$D^{*}\in[4.0,100.0]\times10^{-3}mm^2/s$$$ and applying the relative signal variation from the calibration scans. At each step the action that resulted in the lowest average relative fit error with respect to the drawn ground truth was chosen. One exception was that the number of averages for b-values 0, 150 and 500 s/mm2 was not allowed to reach zero to enable baseline ADC calculations7. Four different scenarios were investigated: pixel-wise fitting vs fitting to the average values within an ROI of size 25 pixels, combined with either a full 4-parameter IVIM fit or a fit where D* was fixed to 40x10-3mm2/s.
Results
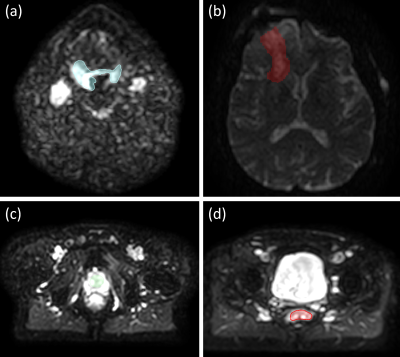
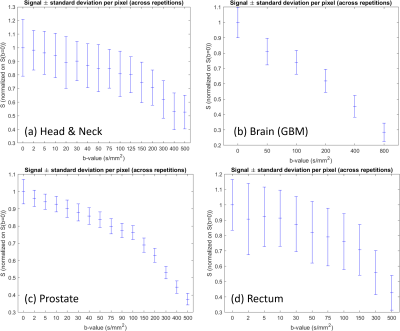
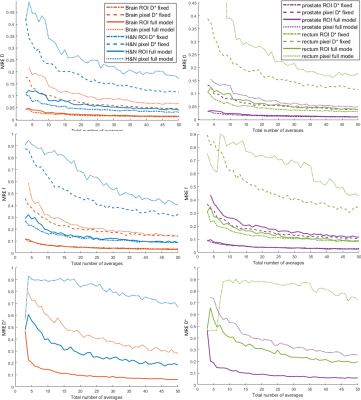
Fig. 1 shows exemplary diffusion-weighted calibration scan images and corresponding contours. The per-pixel standard deviation was calculated across the five repetitions and averaged within the ROI, resulting in the input for the Monte-Carlo simulations (Fig. 2). Fig. 3 displays the dependence of the mean relative error (MRE) on the total number of averages for the different IVIM parameters and anatomical sites. For all anatomical sites MRE D < MRE f < MRE D*. The MRE is strongly reduced for fitting the model to ROI-averaged data, compared to pixel-wise fitting. Table 2 lists the optimal b-values and simulated mean relative errors for a 5 min acquisition, which is acceptable for integration into a clinical MR-Linac treatment workflow.Discussion
Fitting of the full IVIM model for an ROI is possible, but one should consider that the MRE and the variation of the QIBs might depend heavily on the used protocol, when setting up clinical studies. For the range of protocols investigated here, pixel-wise D* mapping led to higher errors than fixing D* to a pre-determined value and could explain why only population average results showed a significant trend in5.For full IVIM model fits, the simulation identified 4-5 different b-values as optimal, while a fit with fixed D* is optimally performed with 3 b-values. MR-Linac systems currently have limitations in terms of gradient performance and qMRI techniques require careful optimization.
Lower resolution could reduce the impact of signal variation but could introduce Gibbs-ringing artifacts and partial volume effects. Deep learning9 or denoising techniques10 could potentially overcome the current limitations regarding D* mapping on MR-Linacs.
The here described technique based on calibration scans could potentially be extended to other QIBs. Future work will investigate repeatability of the optimized IVIM protocols to inform clinical trials.
Conclusion
Application of data-driven techniques for standardizing IVIM protocols is the first step towards reliable estimation of IVIM-derived QIBs in the clinical context of treatment adaptation, response assessment and outcome prediction.Acknowledgements
No acknowledgement found.References
1Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988 Aug;168(2):497-505.
2van Houdt PJ, Saeed H, Thorwarth D, Fuller CD, Hall WA, McDonald BA, Shukla-Dave A, Kooreman ES, Philippens MEP, van Lier ALHMW, Keesman R, Mahmood F, Coolens C, Stanescu T, Wang J, Tyagi N, Wetscherek A, van der Heide UA. Integration of quantitative imaging biomarkers in clinical trials for MR-guided radiotherapy: Conceptual guidance for multicentre studies from the MR-Linac Consortium Imaging Biomarker Working Group. Eur J Cancer. 2021 Aug;153:64-71.
3Lawrence LSP, Chan RW, Chen H, Keller B, Stewart J, Ruschin M, Chugh B, Campbell M, Theriault A, Stanisz GJ, MacKenzie S, Myrehaug S, Detsky J, Maralani PJ, Tseng CL, Czarnota GJ, Sahgal A, Lau AZ. Accuracy and precision of apparent diffusion coefficient measurements on a 1.5 T MR-Linac in central nervous system tumour patients. Radiother Oncol. 2021 Sep 28;164:155-162.
4Paudyal R, Oh JH, Riaz N, Venigalla P, Li J, Hatzoglou V, Leeman J, Nunez DA, Lu Y, Deasy JO, Lee N, Shukla-Dave A. Intravoxel incoherent motion diffusion-weighted MRI during chemoradiation therapy to characterize and monitor treatment response in human papillomavirus head and neck squamous cell carcinoma. J Magn Reson Imaging. 2017 Apr;45(4):1013-1023.
5Kooreman ES, van Houdt PJ, Keesman R, van Pelt VWJ, Nowee ME, Pos F, Sikorska K, Wetscherek A, Müller AC, Thorwarth D, Tree AC, van der Heide UA. Daily Intravoxel Incoherent Motion (IVIM) In Prostate Cancer Patients During MR-Guided Radiotherapy-A Multicenter Study. Front Oncol. 2021 Aug 13;11:705964.
6Lagendijk JJ, Raaymakers BW, Raaijmakers AJ, Overweg J, Brown KJ, Kerkhof EM, van der Put RW, Hårdemark B, van Vulpen M, van der Heide UA. MRI/linac integration. Radiother Oncol. 2008 Jan;86(1):25-9.
7Kooreman ES, van Houdt PJ, Keesman R, Pos FJ, van Pelt VWJ, Nowee ME, Wetscherek A, Tijssen RHN, Philippens MEP, Thorwarth D, Wang J, Shukla-Dave A, Hall WA, Paulson ES, van der Heide UA. ADC measurements on the Unity MR-linac - A recommendation on behalf of the Elekta Unity MR-linac consortium. Radiother Oncol. 2020 Dec;153:106-113.
8Merisaari H, Federau C. Signal to noise and b-value analysis for optimal intra-voxel incoherent motion imaging in the brain. PLoS One. 2021 Sep 23;16(9):e0257545.
9Kaandorp MPT, Barbieri S, Klaassen R, van Laarhoven HWM, Crezee H, While PT, Nederveen AJ, Gurney-Champion OJ. Improved unsupervised physics-informed deep learning for intravoxel incoherent motion modeling and evaluation in pancreatic cancer patients. Magn Reson Med. 2021 Oct;86(4):2250-2265.
10Gurney-Champion OJ, Collins DJ, Wetscherek A, Rata M, Klaassen R, van Laarhoven HWM, Harrington KJ, Oelfke U, Orton MR. Principal component analysis fosr fast and model-free denoising of multi b-value diffusion-weighted MR images. Phys Med Biol. 2019 May 17;64(10):105015.
Figures