2213
Single-shot 3T 1H MRS with dual water/lipid VAPOR suppression for intrahepatic acetylcarnitine detection1Department of Radiology & Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 2Department of Nutrition & Movement Sciences, University of Maastricht, Maastricht, Netherlands
Synopsis
We have developed a novel 3T 1H-MRS sequence to detect acetylcarnitine. Acetylcarnitine has been investigated by 1H-MRS in the muscle and is thought to play an important role in maintaining metabolic flexibility and insulin sensitivity. As these are hallmarks of metabolic disease, techniques to study acetylcarnitine levels non-invasively are of great interest. The advantage of our approach is the absence of a subtraction scheme and high signal intensity, while both water and lipid resonances are sufficiently suppressed to uncover the acetylcarnitine resonance. This makes our sequence suitable also for detection in tissues susceptible to respiratory motion, such as the liver.
Introduction
The conversion of excessive acetyl-coA into acetylcarnitine has been suggested to play an important role in maintaining metabolic flexibility and insulin sensitivity under conditions of excessive substrate supply to the mitochondria.1,2 As decreased metabolic flexibility and insulin sensitivity are early indicators of the development of metabolic disease, such as type 2 diabetes mellitus (T2D)3 and non-alcoholic fatty liver disease (NAFL)4, the development of non-invasive techniques to quantitatively assess acetylcarnitine concentrations in vivo, are of great interest. We previously quantified acetylcarnitine levels by 3T 1H-MRS in skeletal muscle of healthy and metabolically compromised individuals5,6. Our results in skeletal muscle clearly support the premise that acetylcarnitine concentrations associate with insulin sensitivity. To further unravel the role of acetylcarnitine in the development of metabolic diseases, we sought to develop a 1H-MRS sequence that enables the study of this metabolite in the liver. Reliable detection of acetylcarnitine by MRS techniques is hampered by overlapping broad lipid resonances, Our previously developed editing sequences made use of differences in spin-lattice (T1) and transversal (T2) relaxation times between acetylcarnitine and lipids5,6. The most effective lipid suppression was obtained by a subtraction-based T1 editing approach with a long TE of 350ms6. However, as the liver moves with respiration, such a subtraction-based method is sensitive to motion-induced artifacts and therefore infeasible. Here, we propose a single-shot approach with dual water/lipid suppression based on the variable pulse power and optimized relaxation delays (VAPOR) scheme.Methods
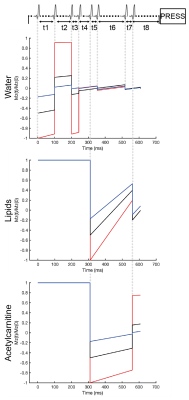
The VAPOR scheme is originally designed to only suppress water7. The advantage of the VAPOR sequence is its relative insensitivity to inhomogeneities in transmit B1 field of the RF coil and variations in T1. The original sequence consists of eight frequency-selective RF pulses of which the timing and flip angles were optimized to null a large range of water T17. To get dual water and lipid suppression, two of the RF pulses were exchanged for inversion pulses (BW75Hz) of which the frequency was set at the acetylcarnitine region. The timing of the two inversion pulses and the timing and relative flip angles of the remaining six VAPOR pulses were optimized through computer simulations in MATLAB (MATLAB 2018b, The MathWorks,Inc.). The voxel used was 40mm x 20mm x 60mm. All spectra were acquired with a TR of 7500ms, TE of 266ms, number of acquired data points of 2048, and number of averages (NSA) of 16. The TE equals 2/Jα-carbonyl and was chosen to refocus J-coupling of α-carbonyl, the resonance closest to acetylcarnitine. The optimized sequence with the simulations are depicted in figure 1. To test the MRS sequence, we acquired 1H-MRS spectra (Achieva 3T MRI, Philips Health Care) of phantoms with 10% intralipid (a soybean oil emulsion (Fresenius Kabi)) in physiological salt including 0mM, 2mM, or 5mM of acetylcarnitine (Sigma Aldrich) and 2% agar (Invitrogen). A regular PRESS with a TE of 266ms and without water and lipid suppression was acquired to use the unsuppressed water signal as an internal reference. To assess the performance of our newly developed sequence (iVAPOR) in comparison to the formerly established sequences for the muscle, we also acquired spectra with the subtraction-based T1 editing sequence with a TE of 350ms6. All obtained spectra were post-processed in a custom-written MATLAB script as previously described8. Acetylcarnitine content was calculated after T2 correction relative to the unsuppressed water resonance.Results
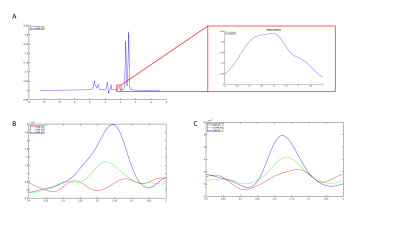
The PRESS spectrum of the phantom with 10% intralipid and 5mM acetylcarnitine (figure 2a) clearly demonstrates the broad lipid resonances that overlap the acetylcarnitine resonance when no lipid suppression is applied. The acetylcarnitine peaks could be revealed in the phantoms containing 2mM and 5mM acetylcarnitine by supressing the lipid resonances using either the subtraction-based T1 editing sequence (figure 2b) or the iVAPOR sequence (figure 2c). Due to the lower TE of the iVAPOR sequence, the signal intensity of the acetylcarnitine peak was higher (figure 2c) when compared with the acetylcarnitine peak obtained with the subtraction-based T1 editing sequence (figure 2b). Integration of the peaks resulted in comparable values when corrected for T2 decay, indicating that both methods work effectively. Measured acetylcarnitine levels by the iVAPOR sequence strongly correlated with the known phantom concentrations.Discussion and Conclusion
Our iVAPOR approach allows the detection of acetylcarnitine in a single-shot, circumventing subtraction artifacts associated with our T1 editing sequence6. The use of inversion pulses makes the iVAPOR approach intrinsically more sensitive to variations in T1 relaxation times, though the absence of a subtraction scheme and a higher signal intensity make this sequence more suitable for detection in tissues susceptible to respiratory motion, such as the liver. Future studies should focus on reproducibility measurements and in vivo validation steps in the muscle and liver.Acknowledgements
No acknowledgement found.References
1. Noland RC, Koves TR, Seiler SE, et al. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem. 2009;284(34):22840-22852. doi:10.1074/jbc.M109.032888
2. Muoio DM, Noland RC, Kovalik JP, et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012;15(5):764-777. doi:10.1016/j.cmet.2012.04.005
3. Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23(7):804-818. doi:10.1038/nm.4350.Insulin
4. Manco M. Insulin Resistance and NAFLD: A Dangerous Liaison beyond the Genetics. Children. 2017;4(8):74. doi:10.3390/children4080074
5. Lindeboom L, Nabuurs CI, Hoeks J, et al. Long-echo time MR spectroscopy for skeletal muscle acetylcarnitine detection. J Clin Invest. 2014;124(11):4915-4925. doi:10.1172/JCI74830
6. Lindeboom L, Bruls YMH, van Ewijk PA, et al. Longitudinal relaxation time editing for acetylcarnitine detection with 1H-MRS. Magn Reson Med. 2017;77(2):505-510. doi:10.1002/mrm.26149
7. Tkáč I, Deelchand D, Dreher W, et al. Water and lipid suppression techniques for advanced 1H MRS and MRSI of the human brain: Experts’ consensus recommendations. NMR Biomed. 2021;34(5):1-25. doi:10.1002/nbm.4459
8. Roumans KHM, Lindeboom L, Veeraiah P, et al. Hepatic saturated fatty acid fraction is associated with de novo lipogenesis and hepatic insulin resistance. Nat Commun. 2020;11(1):1-11. doi:10.1038/s41467-020-15684-0
Figures