2212
Hyperpolarized 129Xe MRI is sensitive to variations in gas exchange impairment in patients with long haul COVID-191Radiology, Duke University, Durham, NC, United States, 2Biomedical Engineering, Duke University, Durham, NC, United States, 3Medical Physics, Duke University, Durham, NC, United States, 4Physics, Duke University, Durham, NC, United States, 5Bioengineering, McGill University, Montreal, QC, Canada, 6Medicine, Duke University, Durham, NC, United States
Synopsis
Many patients who recover from COVID-19 have symptoms that persist long after the acute phase of the disease has subsided, including fatigue and dyspnea on exertion. There is an unmet need for understanding the diverse cardiac and pulmonary causes of these symptoms and to monitor patients longitudinally. We imaged N=6 subjects with long-haul COVID using both cardiac MRI and hyperpolarized 129Xe MRI and spectroscopy. While cardiac imaging did not reveal structural or functional abnormalities, we observed a wide variety of findings on 129Xe MRI, with subjects who had been hospitalized exhibiting the most pronounced gas exchange impairment.
INTRODUCTION
Evidence is mounting that many patients who have recovered from acute COVID-19 have residual cardiopulmonary disease in the form of persistent, disabling symptoms characterized as “long-haul COVID.” As the causes for dyspnea are multifactorial, there is an urgent need to not only elucidate potential cardiac and pulmonary causes, but also to monitor them longitudinally and non-invasively. Here, we used dual MR imaging (cardiac MRI and hyperpolarized 129Xe MRI) to characterize the impact of long-haul COVID-19 on cardiac and regional gas exchange function.METHODS
Patients with persistent and continued dyspnea at least 50 days after initial COVID-19 diagnosis (N=6) underwent hyperpolarized 129Xe MRI and spectroscopy together with cardiac MRI (including T1 and T2 mapping and late gadolinium enhancement). For each patient, signals from gas phase (airspaces) and dissolved phase (interstitial barrier tissue uptake and red blood cell [RBC] transfer) were acquired during a single breath-hold1. Functional image voxels were binned according to thresholds derived from a healthy reference cohort2. The ratio of RBC to barrier signal (RBC:Barrier) was obtained from 129Xe spectroscopy3.RESULTS
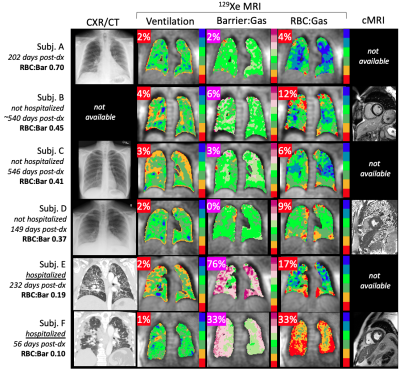
Patients underwent 129Xe imaging a median of 232 days (min 56, max 546) following initial COVID-19 diagnosis. Ventilation was uniformly well preserved (Figure 1), with a ventilation defect percent mean±SD of 2.3±1.0% (healthy reference 5±3%). However, high barrier percent varied widely, from 0% to 76% (overall 20.0±30.0%, healthy reference 1±1%). Most subjects exhibited some degree of RBC defect (13.5±10.6%, healthy reference 4±2%), as well as reduced RBC:Bar (0.37±0.21, healthy reference 0.57±0.07). No abnormalities were observed on available cardiac MRI. Although all patients reported some combination of persistent dyspnea/fatigue, imaging reveals some to have significantly impaired gas exchange function while in others it was relatively preserved. Notably, the two patients with elevated high barrier percent were the only two patients who had been hospitalized.DISCUSSION
Subjects with persistent chronic respiratory symptoms exhibited a striking variety of findings on 129Xe MRI, ranging from apparently normal lung function to severe gas exchange impairment. These preliminary results demonstrate the utility of 129Xe MRI for evaluating pulmonary function and differentiating subtypes in long-haul COVID-19. Subjects who had not been hospitalized (Subjects A-D in Figure 1) exhibited relatively well-preserved gas exchange function. In contrast, both of the patients who had been hospitalized (Subjects E and F) exhibited high barrier uptake and reduced RBC:Barrier at the 129Xe MRI visit despite being months removed from their initial COVID-19 diagnosis. This suggests the presence of a persistent but potentially reversible degree of high barrier uptake, as has also been observed in interstitial lung disease4,5. However, only Subject F exhibited a marked RBC defect abnormality, possibly reflecting irreversible vascular damage.This study population will return for a six-month follow-up imaging visit, enabling the evaluation of longitudinal changes in 129Xe findings in the context of therapeutic intervention and disease progression.
CONCLUSION
In patients with persistent chronic symptoms of COVID-19, 129Xe MRI may be useful as a sensitive means of assessing gas exchange abnormalities, longitudinal disease monitoring, and evaluating treatment response.Acknowledgements
Grant supported by Translating Duke Health Cardiovascular Disease Initiative-COVID-19 (co:PI’s: Que/Driehuys).References
1. Niedbalski PJ, Hall CS, Castro M, et al. Protocols for multi‐site trials using hyperpolarized 129Xe MRI for imaging of ventilation, alveolar‐airspace size, and gas exchange: A position paper from the 129Xe MRI clinical trials consortium. Magn Reson Med 2021;
2. Wang Z, Robertson SH, Wang J, et al. Quantitative analysis of hyperpolarized 129Xe gas transfer MRI. Med Phys 2017;44(6):2415–2428.
3. Bier EA, Robertson SH, Schrank GM, et al. A protocol for quantifying cardiogenic oscillations in dynamic 129Xe gas exchange spectroscopy: The effects of idiopathic pulmonary fibrosis. NMR Biomed 2019;32(1):e4029.
4. Rankine LJ, Wang Z, Wang JM, et al. 129Xenon Gas Exchange Magnetic Resonance Imaging as a Potential Prognostic Marker for Progression of Idiopathic Pulmonary Fibrosis. Ann Am Thorac Soc 2020;17(1):121–125.
5. Mummy DG, Bier EA, Wang Z, et al. Hyperpolarized 129Xe MRI and Spectroscopy of Gas-Exchange Abnormalities in Nonspecific Interstitial Pneumonia. Radiology 2021;301(1):211–220.
Figures