2208
Post-Acute COVID-19 Syndrome: Longitudinal 129Xe MRI Ventilation Heterogeneity Measurements1Robarts Research Institute, London, ON, Canada, 2Department of Medical Biophysics, Western University, London, ON, Canada, 3Department of Physics, Ryerson University, Toronto, ON, Canada, 4Division of Respirology, Department of Medicine, Western University, London, ON, Canada
Synopsis
Post-acute COVID-19 syndrome (PACS) is an umbrella term for the long-term symptoms and poor quality-of-life that follow acute SARS-CoV-2 viral-infection in up to 7/10 COVID-19 survivors. The mechanistic understanding of PACS remains poorly understood, which stymies treatment decision-making. In a PACS pilot study, we evaluated hyperpolarized 129Xe MRI ventilation defect percent and texture features to measure potential longitudinal changes. There were no significant differences in 129Xe MRI ventilation texture measurements six-months and 12-months after a baseline visit (12 weeks post-infection). PACS participants exhibited abnormal second-order 129Xe MRI ventilation textures and patchy ventilation, relative to healthy controls, 6-12 months post-infection.
INTRODUCTION
Post-acute COVID-19 syndrome (PACS) is an umbrella term for the long-term symptoms and poor quality-of-life that follows the acute COVID-19 infectious disease phase in approximately 73% of patients.1,2 However, the mechanisms and pathologies behind the pulmonary sequelae of COVID-19 are not well-understood, and this makes treatment planning and decision-making very challenging.Hyperpolarized 129Xe magnetic resonance imaging (MRI) ventilation defect percent (VDP) quantifies inhaled gas distribution abnormalities.3 129Xe MRI ventilation texture features extracted from gray-level-run-length-matrices (GLRLM) can be used to quantify the differences in signal intensity across the ventilated regions of the lung.4 From these matrices, second-order texture features, such as short run emphasis (SRE), high gray-level run emphasis (HGRE), short-run high gray-level run emphasis (SRHGE), and short-run low gray-level run emphasis (SRLGE), have been used to evaluate ventilation heterogeneity.5
Hyperpolarized 129Xe MRI texture features may provide additional information about the abnormal ventilation associated with the persistent sequelae in PACS. Therefore, the objective of this pilot study was to evaluate potential longitudinal changes in ventilation texture features in PACS.
METHODS
We evaluated 17 participants with persistent respiratory symptoms, who completed a baseline visit three months post-infection and a six-month or 12-month follow-up. All participants provided written informed consent to an approved protocol that included thoracic CT, MRI, and pulmonary function testing. The participants attended follow-up visits either at six-months (n=10) or 12-months (n=7) following their initial, baseline visit for repeat 129Xe MRI and pulmonary function testing. CT imaging at baseline was acquired in nine participants. A total of nine contemporaneous healthy volunteers were evaluated at baseline in the control arm of this study.Anatomical 1H and hyperpolarized 129Xe MRI were acquired using a 3.0 Tesla Discovery MR750 (General Electric Health Care, WI, USA) with broadband capability as previously described.6,7 Anatomical proton images were acquired using a fast-spoiled gradient-recalled echo sequence (total acquisition time, 8 seconds; TR msec/TE msec, 4.7/1.2; flip angle, 30°; field of view, 40×40 cm2; bandwidth, 24.4 kHz; 128×80 matrix, zero padded to 128×128; partial echo percentage, 62.5%; 15-17 sections; section thickness, 15 mm; no gap). Static ventilation images were acquired using a three-dimensional fast gradient-recalled echo sequence (total acquisition time, 14 s; TR msec/TE msec, 6.7/1.5; variable flip angle; field of view, 40×40 cm2; bandwidth, 15.63 kHz; 128×128 matrix; 14 sections; section thickness, 15 mm; no gap). Participants were instructed to inhale 1.0 L of gas (100% N2 for anatomical scan and 400 mL hyperpolarized 129Xe mixed with 600 mL 4He for hyperpolarized scan) to ensure volume matched images for segmentation. MRI was acquired under breath hold conditions. Images were segmented and VDP was quantified using a semi-automatic method and cluster analysis tools.3
CT images were acquired using a 640 slice LightSpeed VCT system (General Electric Healthcare) during breath-hold of 1.0L of N2 gas (64×0.625 collimation, 129 peak kilovoltage, 100mA, tube rotation time 500ms, pitch 1.25, standard reconstruction kernel, 1.25mm slice thickness, FOV=40cm2).
We used the GLRLM method, modified for 129Xe MRI texture feature analysis, to calculate 11 second-order texture features.4,5 The signal-to-noise ratio (SNR) of each 129Xe MRI slice was determined such that the texture features were averaged for only those slices with a SNR value greater than 15. Thoracic CT images were analyzed using VIDAvision software (VIDA Diagnostics Inc., Coralville, IA, USA) to segment airways.
Paired samples t-tests were used to evaluate the differences in MRI ventilation texture features in PACS participants at two time points. Independent samples t-tests were used to evaluate the differences between PACS and healthy control participants.
RESULTS
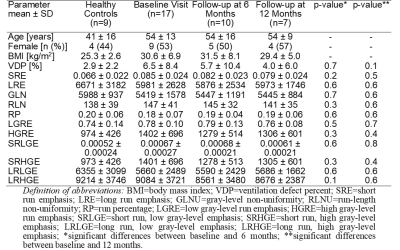
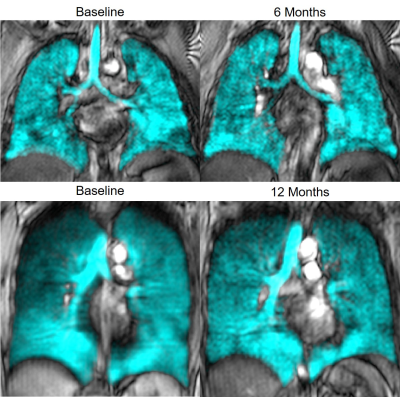
Table 1 provides demographic and imaging characteristics for healthy controls and for PACS participants at baseline and follow-up visits. Figure 1 illustrates the qualitative similarities in 129Xe MRI ventilation patterns between the time points. There were no significant differences in second-order texture features and VDP between initial and follow-up visits for PACS participants. Similar to previous findings,8 PACS participants at both follow-up points were significantly different from healthy controls in SRE, HGRE, and SRHGE, while only participants at the six-month time point had significant differences in SRLGE. We observed quantitative CT airway measurements of total airway count, wall area, and lumen area in PACS are comparable to literature values for healthy participants.9,10DISCUSSION
129Xe MRI ventilation patterns and texture features were not qualitatively different, six or 12-months following a baseline visit. The fact that ventilation texture and VDP did not change over time in PACS participants is consistent with persistent dyspnea and other limitations that are the hallmark of PACS. At both time points, heterogeneous ventilation and ventilation patchiness were abnormal. 129Xe MRI ventilation texture features in PACS were significantly different relative to healthy controls, despite having normal quantitative CT airway measurements.CONCLUSION
Second-order 129Xe MRI ventilation texture features were abnormal in PACS participants relative to non-infected healthy controls, six-months and 12-months post-baseline visit, while baseline CT measurements were normal. Texture features that quantify patchy ventilation in PACS participants did not significantly differ between baseline and follow-up, suggesting 129Xe MRI patchy ventilation characterizes pulmonary abnormalities in PACS and that this persists over long periods of time.Acknowledgements
No acknowledgement found.References
1 Nalbandian, A. et al. Nat Med (2021).
2 Nasserie, T. et al. JAMA Netw Open (2021).
3 Kirby, M. et al. Acad Radiol (2012).
4 Haralick, R.M. et al. IEEE Trans. Syst Man Cybern Syst (1973).
5 Zha, N. et al. Acad Radiol (2016).
6 Parraga, G. et al. Invest Radiol (2007).
7 Svenningsen, S. et al. J Magn Reson Imaging (2013).
8 Kooner, H.K. et al. Proc ISMRM (2020).
9 Kirby, M. et al. Am J Respir Crit Care Med (2018).
10 Zach, J.A. et al. Invest Radiol (2012).